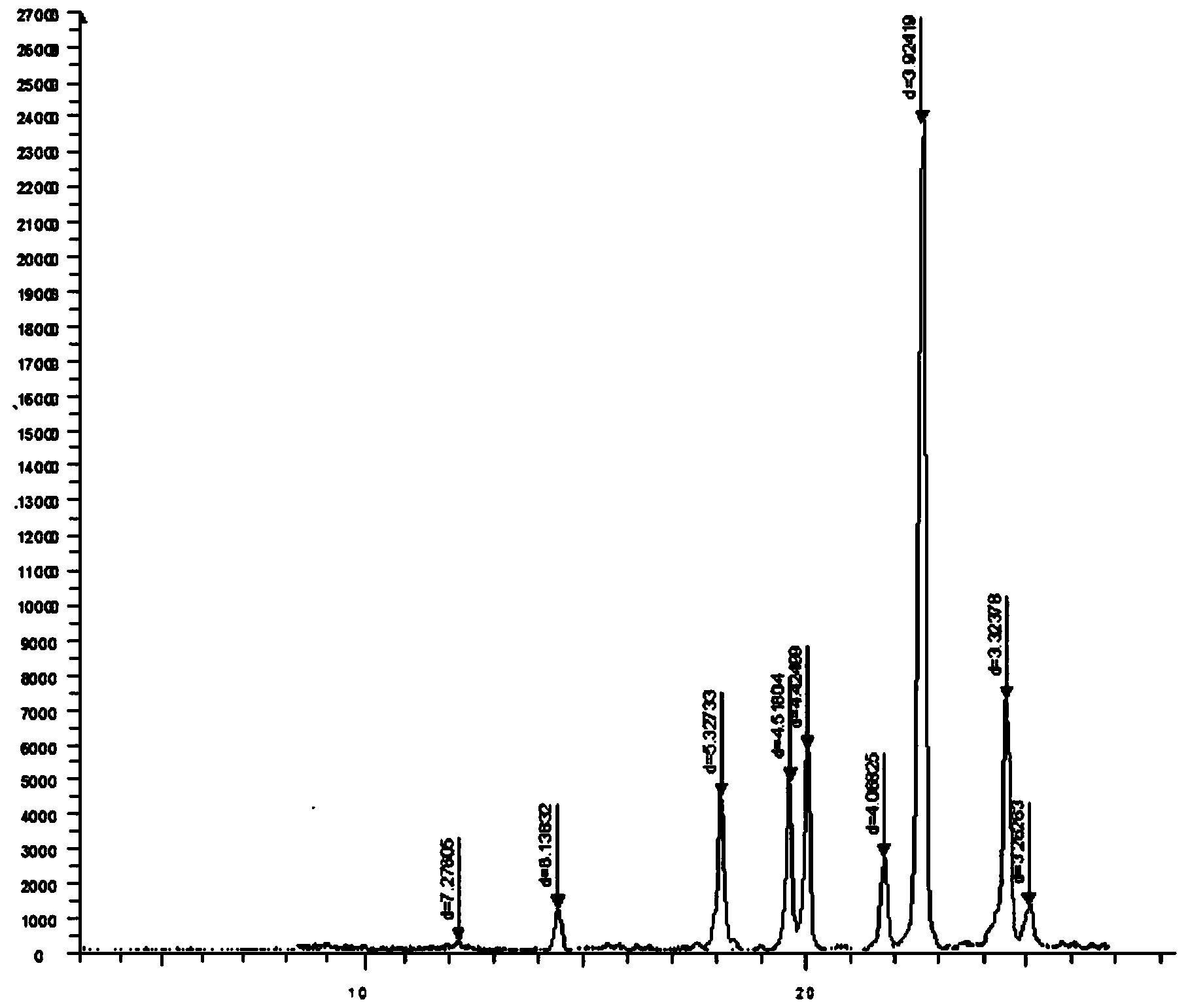
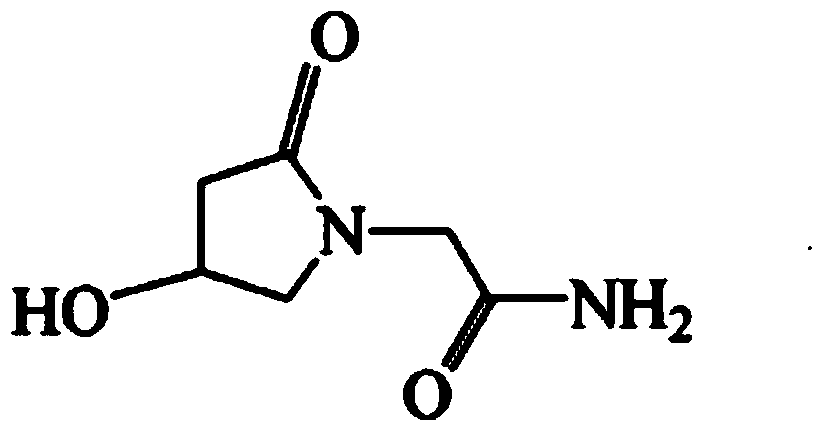
Oxiracetam compound and pharmaceutical composition thereof
A compound, alpha ray technology, applied in the field of medicine, can solve the problems of poor quality stability, decreased dissolution, and increased impurity content, and achieves the effects of good stability, good stability and high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] 1) Completely dissolve 50g of oxiracetam in a mixed solvent of 600ml of tetrahydrofuran and 300ml of ethylene glycol, stir until it is completely dissolved, adjust the pH value of the liquid to 5 with acetic acid, then add activated carbon, stir for adsorption, filter to remove carbon Bacteria, to obtain a clear solution, move the clear solution into a pressure vessel, and under the condition that the pressure in the pressure vessel is 1Mpa and the temperature of the clear solution in the pressure vessel is 60°C, slowly add 300ml of isopropyl ether to produce a white precipitate, filter , washed successively with methanol and deionized water, and dried under reduced pressure to obtain the oxiracetam compound in the form of white microcrystalline powder;
[0049] 2) Add the oxiracetam crystals obtained in step 1) into toluene and heat up to 65°C, heat it for 8 hours and filter while it is hot, and then fine-filter the filtrate with a 0.45 μm microporous membrane;
[0050...
Embodiment 2
[0059] 1) Completely dissolve 50 g of oxiracetam in a mixed solvent of 675 ml of tetrahydrofuran and 225 ml of ethylene glycol, adjust the pH value of the liquid to 6.5 with acetic acid, then add activated carbon, stir for adsorption, filter for decarburization and sterilize to obtain a clear solution, Move the clarified solution into a pressure vessel, and slowly add 250ml of isopropyl ether under the condition that the pressure in the pressure vessel is 1Mpa and the temperature of the clarified solution in the pressure vessel is 70°C, to produce a white precipitate, filter it, and then use methanol, then remove Washing with deionized water and drying under reduced pressure to obtain the white microcrystalline powder oxiracetam compound;
[0060] 2) Add the oxiracetam crystals obtained in step 1) into toluene and heat up to 80°C, keep warm for 3 hours and filter while it is hot, and then fine filter the filtrate with a 0.45um microporous membrane;
[0061] 3) Collect the filt...
Embodiment 3
[0067] Completely dissolve 50g of oxiracetam in a mixed solvent of 500ml of tetrahydrofuran and 400ml of ethylene glycol, adjust the pH value of the liquid to 5.5 with acetic acid, then add activated carbon, stir for adsorption, filter for decarbonization and sterilization, and obtain a clear solution. Move the solution into a pressure vessel, and under the condition that the pressure in the pressure vessel is 1Mpa and the temperature of the clarified solution in the pressure vessel is 65°C, slowly add 350ml of isopropyl ether to produce a white precipitate, filter it, and wash it with methanol and deionized water successively. Wash and dry under reduced pressure to obtain the oxiracetam compound in the form of white microcrystalline powder;
[0068] 2) Add the oxiracetam crystals obtained in step 1) into toluene and heat up to 70°C, heat it for 5 hours and filter it while it is hot, and then fine filter the filtrate with a 0.45um microporous membrane;
[0069] 3) Collect the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com