Preparation method of vinylbenzyl-polyphenyl ether composition
A technology of vinylbenzyl and polyphenylene ether, which is applied in the field of preparation of vinylbenzyl-polyphenylene ether compounds, and can solve the problems of low yield, long engineering time, difficult decompression fractionation and removal, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
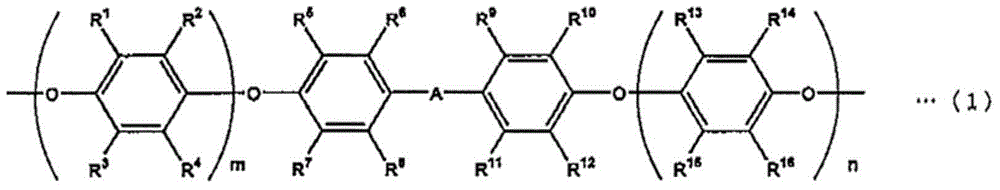
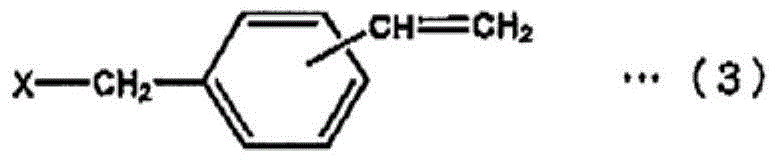
[0073] In a 2L four-neck flask equipped with a temperature regulator, a stirring device, a condenser and a dropping funnel, put 158g (0.1 moles) of polyphenylene ether represented by general formula (1) (R in general formula (1) 1 , R 3 , R 5 , R 7 , R 10 , R 12 , R 14 , R 16 is methyl, R 2 , R 4 , R 6 , R 8 , R 9 , R 11 , R 13 , R 15 hydrogen, A is isopropylidene, m+n=average 12, number average molecular weight is 1580), 221g toluene, 94.8g isopropanol, mix well, then add 0.96g tetra-n-butylammonium bromide, 33.6 g (0.22 mol) of vinyl benzyl chloride (meta body / para body = 50 / 50, trade name: CMS-P, manufactured by Seimichemical Co.), and the temperature was raised to 75°C. Here, 53.3 g (0.64 mol) of 48% sodium hydroxide aqueous solution was dripped over 30 minutes, and the reaction was carried out at 75° C. for 5 hours, and the reaction rate was 98% or more.
[0074] Next, as a step of removing unreacted vinylbenzyl chloride, 16.7 g (2.0 equivalents to 1 equiva...
Embodiment 2
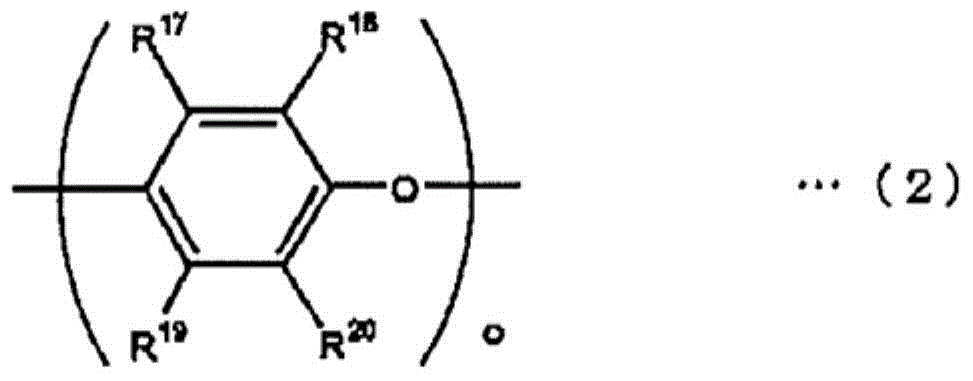
[0077] In a 2L four-necked flask equipped with a temperature regulator, a stirring device, a condenser and a dropping funnel, put 240g (0.1 mol) of polyphenylene ether represented by general formula (2) (R in general formula (2) 17 for hydrogen, R 18 is methyl, R 19 for hydrogen, R 20 Methyl, o=20, number average molecular weight 2400), 336g toluene, 144g isopropanol, mix well, then add 1.30g tetra-n-butylammonium bromide, 19.8g (0.13 mole) vinylbenzyl Chloride (metasite / parasite = 50 / 50, trade name: CMS-P, manufactured by Seimichemical Co.), the temperature was raised to 75°C. Here, 27.5 g (0.33 mol) of 48% sodium hydroxide aqueous solution was dripped over 30 minutes, and the reaction was carried out at 75° C. for 5 hours, and the reaction rate was 98% or more.
[0078]Next, as a step of removing unreacted vinylbenzyl chloride, 16.7 g (2.0 equivalents to 1 equivalent of polyphenylene ether) 48% sodium hydroxide, 6.0 g (to 100 Parts by mass of polyphenylene ether is 2.5 p...
Embodiment 3
[0081] Except that the tetra-n-butylammonium bromide used in the step of removing unreacted vinylbenzyl halide was replaced with 8.0 g (5.0 parts by mass relative to 100 parts by mass of polyphenylene ether), and the same as the implementation Example 1 was carried out in the same manner to obtain 345 g of the product (yield based on polyphenylene ether: 95%). The amount of vinylbenzyl chloride remaining in the obtained product (solid content conversion value, the same below) is less than 0.1%, the halide ion concentration is 4ppm, and the number average molecular weight of vinylbenzyl-polyphenylene ether compound is 2170, The weight average molecular weight was 3650.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com