Converted attenuated listeria introducing EB virus LMP2A nucleotide sequence and vaccine of converted attenuated listeria
A technology of nucleotide sequence and Listeria, which is applied in the field of transforming attenuated Listeria and its vaccine, can solve the problems that are not suitable for treatment and prevention of nasopharyngeal carcinoma, and achieve good prevention and treatment of tumors, high safety and reliability Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Example 1 Preparation method of attenuated Listeria vaccine
[0023] The strain in this example was provided by Professor Franckle of the University of Pennsylvania. This Listeria species is a defective strain, which lacks the D-alanine synthetase that synthesizes the bacterial cell wall and cannot survive independently. The P1565 vector we use contains subtilis D-alanine synthase gene of Bacillus. When the P1565 plasmid vector is introduced, the bacteria can synthesize the D-alanine of Bacillus subtilis, so that the bacteria can survive independently to a certain extent and self-replicate to a limited extent without causing obvious damage to tissues and organs.
[0024] The medium / reagent that embodiment relates to:
[0025] Improved BHI (heart-brain perfusion fluid) medium: 100ml deionized water + 3.7g BHI dry powder + 20mg D-alanine;
[0026] BHIS (sucrose heart and brain perfusion solution): 100ml deionized water + 3.7g BHI dry powder + 17.115g sucrose + 20mg D-...
Embodiment 2
[0049] Example 2 The Second Homologous Recombination Experiment of Attenuated Listeria Secreting Epstein-Barr Virus LMP2A Protein
[0050] After the attenuated Listeria is transferred into the P1565-LMP2A shuttle plasmid by electroporation, if it is to become a qualified vaccine, it must be able to continuously and stably secrete LMP2A protein to effectively activate the body's immune response.
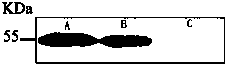
[0051] The present invention continues to culture the second homologous recombination colony obtained in Example 1 in the BHI medium, utilizes the TCA / acetone precipitation method to extract the secreted protein in the BHI medium, and uses the western blot method to identify the protein in the protein expression. Identification, experimental results show that the attenuated Listeria vaccine can stably secrete LMP2A protein, figure 1 Immunoblot of Epstein-Barr virus LMP2A protein secreted for the transformed attenuated Listeria vaccine, wherein A is the protein extracted from CNE-1...
Embodiment 3
[0052] Example 3 To detect the distribution in vivo of the recombined attenuated Listeria vaccine of Example 1, the method is:
[0053] Experimental group: the recombinant attenuated Listeria vaccine provided in Example 1;
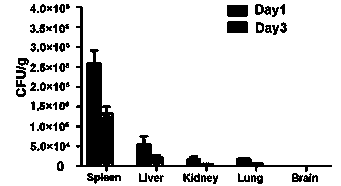
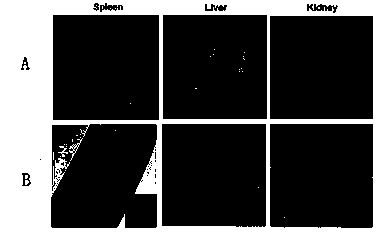
[0054] Select 9 C57BL / 6 mice with a body weight of 10 g, and use the vaccine of the experimental group to immunize the mice three times through tail vein injection, 10 5 CFU / mouse, immunized once a week, for three consecutive weeks; the liver, spleen, lung, and brain of the mice were extracted from the liver, spleen, lung, and brain of the mice on the 1st and 3rd days after the third immunization, and the results showed that the recombinant Listeria Bacteria were mainly distributed in the liver and spleen tissue, a small amount of Listeria was found in the kidney, almost no Listeria was distributed in the lung and brain tissue, and there was no significant statistical difference in the distribution of liver and spleen. content in figure 2 shown. Simult...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com