Medical composition for treating bile related diseases, and preparation method and application thereof
The technology of a composition and medicine is applied in the pharmaceutical composition for the treatment of gallbladder-related diseases, the botanical composition for the treatment of digestive system diseases, and the preparation field, which can solve the problems of patient death and the like, and achieve pain relief, inhibition of normal bile secretion, The effect of suppressing abnormal bile secretion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0078] The steps of the preparation method of the oral liquid of the present invention are as follows:
[0079] Take 4.0-2.0 parts by weight of Phyllanthus emblica, 3.2-0.5 parts by weight of Myrobalan, and 2.8-0.4 parts by weight of Myrobalan, which are cleanly selected, free of impurities, insects, and mildew, and are used after passing the inspection.
[0080] Mix the above components, add 6-20 times the weight of water and decoct 2 times for 1 hour each time, filter, combine the filtrate, and place in a vacuum concentration tank at 60°C and 0.02mpa under reduced pressure and concentrate to a relative density of 1.10- 1.25(20℃) extract, add appropriate amount of agar and honey, mix well, place at 4°C for 48 hours, filter, add appropriate amount of flavoring to the filtrate, mix well, add appropriate amount of Pengrun soil, mix well, place at 4°C for 48 hours, filter repeatedly , The filtrate is sent to the 100,000 clean-level automatic bottling workshop for filling, and each bot...
Embodiment 2
[0100] The steps of the preparation process of the capsule of the present invention are as follows:
[0101] Take 4.0-2.0 parts by weight of Phyllanthus emblica, 3.2-0.5 parts by weight of Myrobalan, and 2.8-0.4 parts by weight of Myrobalan, which are cleanly selected, free of impurities, insects, and mildew, and are used after passing the inspection.
[0102] Mix the above components, add 6-20 times the amount of water and decoct 2 times for 1 hour each time, filter, combine the filtrate, and place in a vacuum concentration tank at 60°C and 0.02mpa under reduced pressure and concentrate to a relative density of 1.10- Add ethanol to the extract at 1.25 (20℃) until the ethanol content reaches 70%, mix well, let stand overnight, filter, and after the filtrate recovers ethanol, place it in a vacuum concentration tank, and concentrate at 60℃ and 0.02mpa under reduced pressure. Thick paste with a density of 1.28-1.40 (20°C). Take starch, dextrin, and powdered sugar (7:1:1) as auxiliary...
Embodiment 3
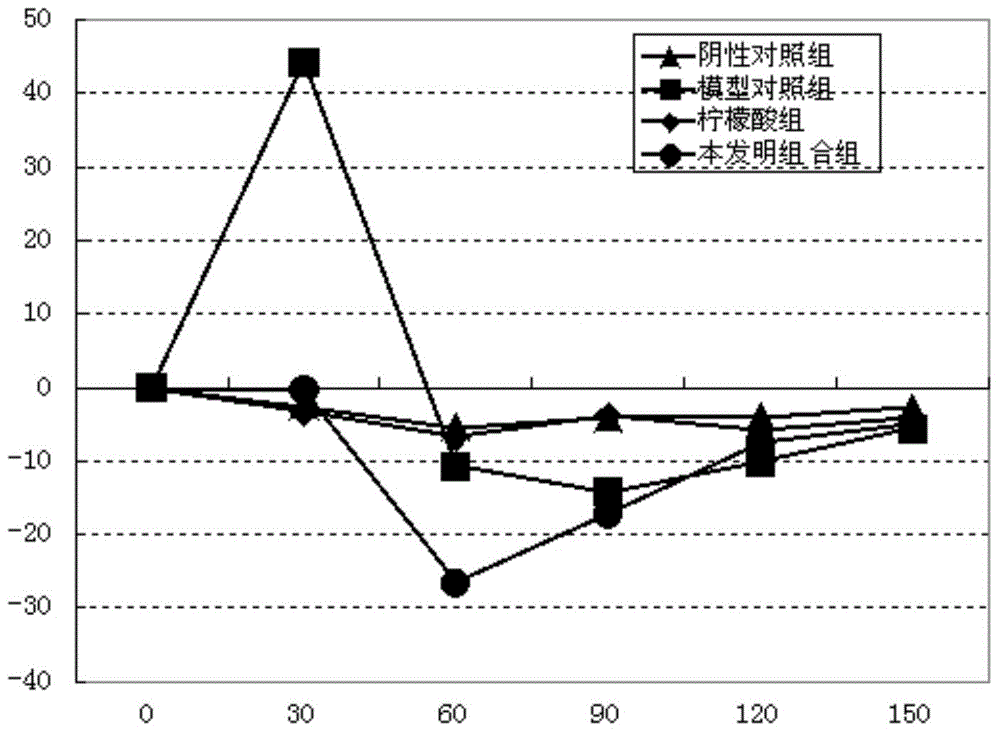
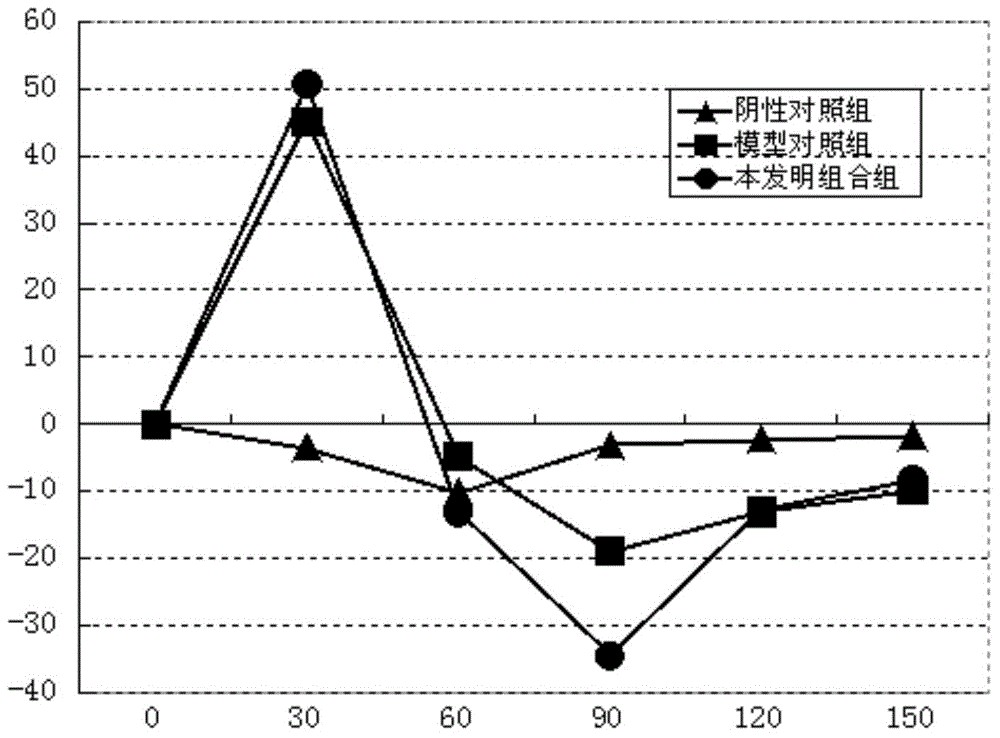
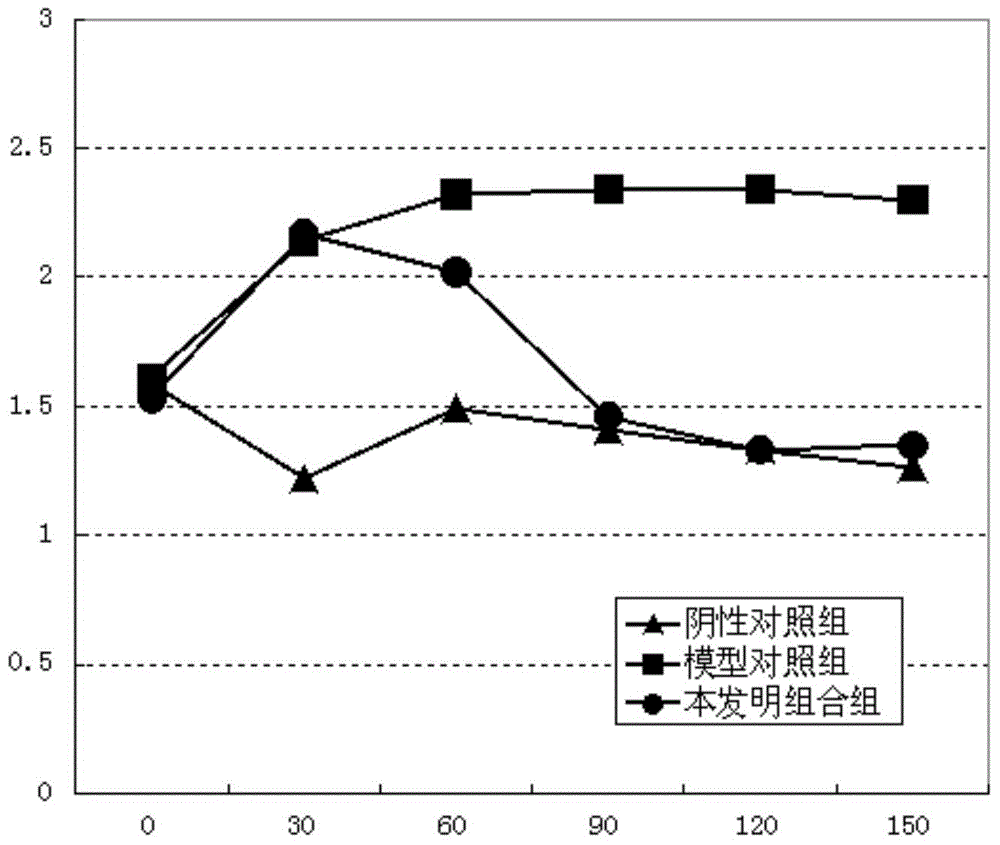
[0115] Example 3. Study on the pharmacological effectiveness of the combination of the present invention
[0116] Experiment 1. The effect of the combination of the present invention on normal bile secretion:
[0117] The guinea pigs were randomly grouped according to their body weight, 10 in each group. Before the experiment, the animals were fasted for 12 hours without water, and the animals in each group were anesthetized by intraperitoneal injection of 20% uracil 1mL / 100g.bw, and then the common bile duct was inserted into the silicone tube. After the operation was completed, the bile was collected and the bile volume was measured to observe the stability of bile secretion. The test substance is based on the clinical dose. The equivalent dose of guinea pigs converted to body surface area is 1.17g crude drug / kg. Take 0.52g crude drug / mL of SLJ oral liquid and administer 1mL / 100g, which is 5.2g crude drug / kg. After the bile secretion stabilized (90 minutes), the test substance...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com