Phenylpropionic acid compounds chained by nitrogen-containing heterocyclic rings, pharmaceutical composition, preparation method, and application thereof
A technology of phenylpropionic acids and compounds, applied in the field of pharmaceuticals, can solve the problems of insufficient insulin secretion function of pancreatic beta cells, collapse of compensatory mechanisms, and elevated blood sugar levels.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
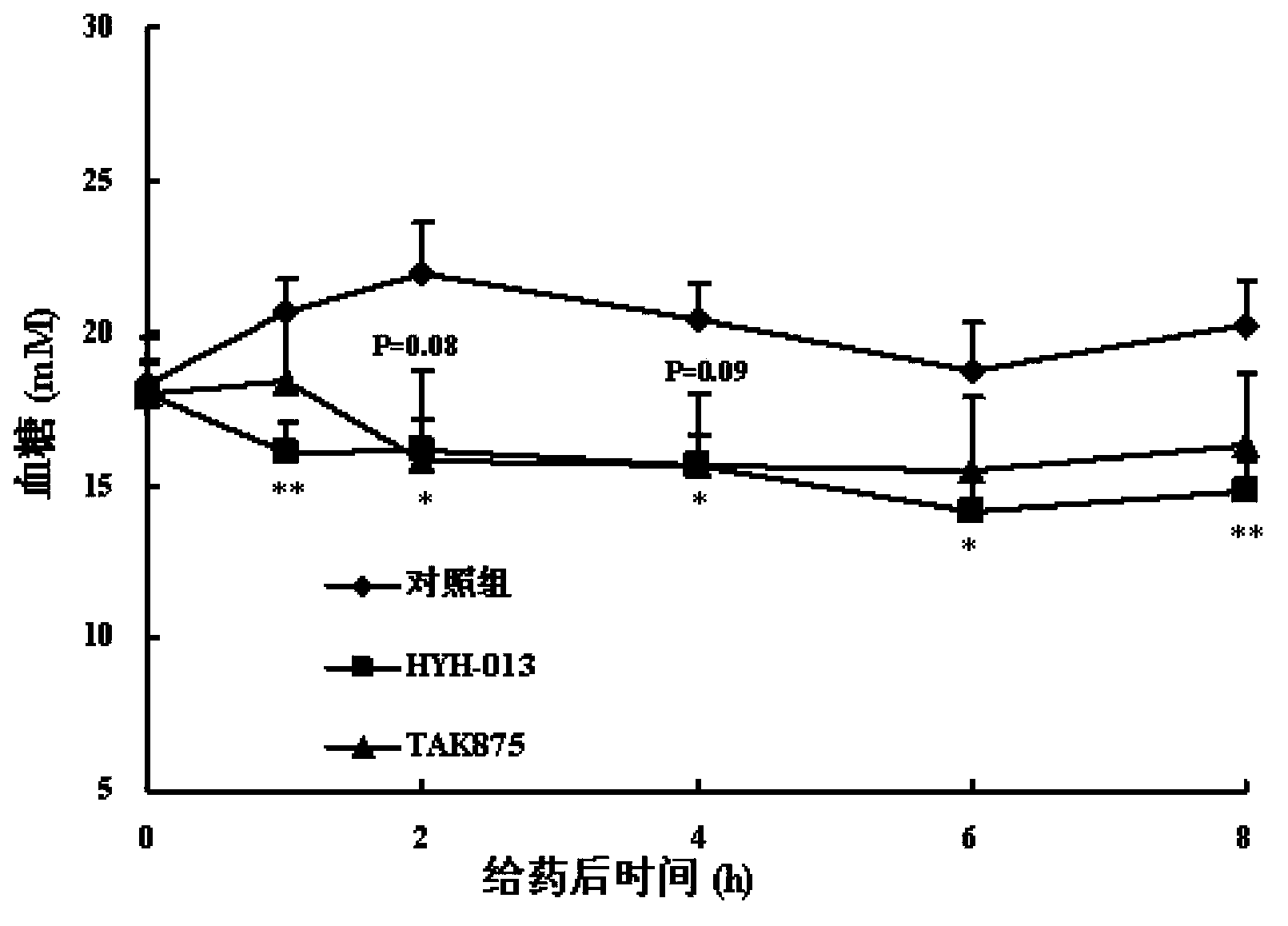
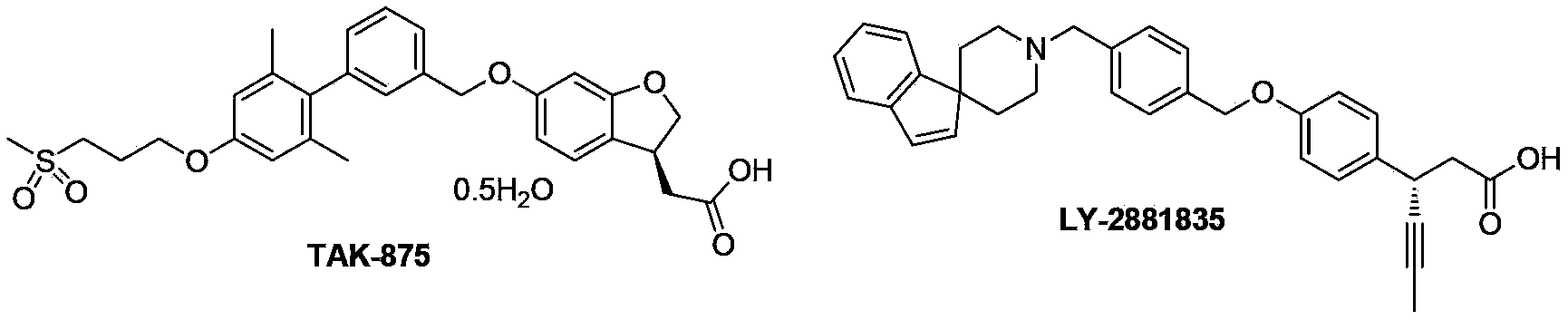
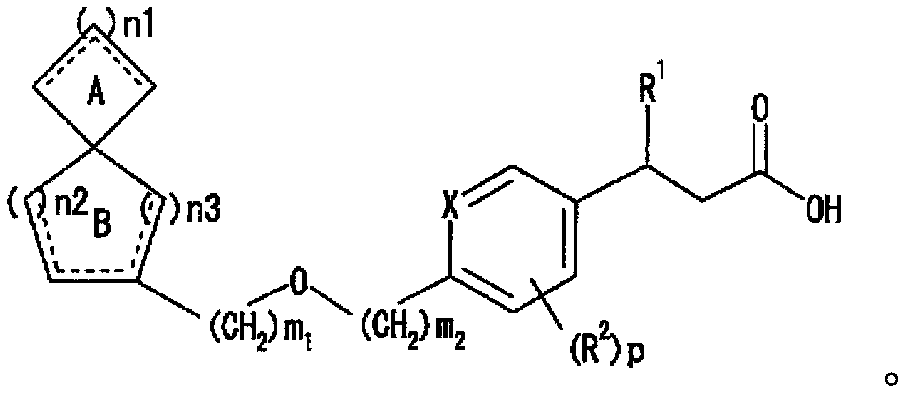
Image
Examples
Embodiment 1
[0081] Embodiment 1: the preparation of 3-(4-bromomethylphenyl) ethyl propionate
[0082]
[0083] Weigh 4-bromobenzyl alcohol 1a (purchased from Shaoyuan Chemical Technology (Shanghai) Co., Ltd., 10.50 g, 56.0 mmol,) and dissolve it in 100 mL of triethylamine, add 20.0 mL of ethyl acrylate 2, palladium acetate (0.50 g, 2.2mmol), triphenylphosphine (0.50g, 1.9mmol). The reaction system was replaced with nitrogen three times, then placed in an oil bath at 95°C for 72 hours, the reaction solution was cooled to room temperature, filtered, and the filtrate was concentrated, then separated by silica gel column chromatography (petroleum ether / ethyl acetate=4 / 1) to obtain a white Solid 3a 10.8 g, 93% yield. 1 H NMR (300MHz, CDCl 3 ): δ=7.67(d,J=16.0Hz,1H),7.51(d,J=8.1Hz,2H),7.38(d,J=8.1Hz,2H),6.42(d,J=16.0Hz,1H ), 4.72(d, J=5.4Hz, 2H), 4.26(q, J=7.2Hz, 2H), 1.33(t, J=7.1Hz, 3H).
[0084] Dissolve 10.8g of intermediate 3a in 100mL of ethyl acetate, add 200mg of 10% palladium ca...
Embodiment 2
[0086] Embodiment 2: Preparation of 3-(4-bromomethyl-2-fluorophenyl) ethyl propionate
[0087]
[0088] Compound 3-(4-bromomethyl-2-fluorophenyl) ethyl propionate (compound 5b) was prepared according to a method similar to that shown in Example 1, substituting compound 1b for compound 1a. 1 H NMR (300MHz, CDCl 3 )δ7.19(t,J=7.8Hz,1H),7.11–7.03(m,2H),4.43(s,2H),4.12(q,J=7.1Hz,2H),2.96(t,J=7.6 Hz,2H),2.61(t,J=7.6Hz,2H),1.23(t,J=7.1Hz,3H).
Embodiment 3
[0089] Embodiment 3: Preparation of ethyl 3-(4-(((6-bromopyridin-2-yl)oxy)methylene)phenyl)propionate
[0090]
[0091] Weigh 2.50g of compound 6a, 3.90g of compound 5a, and 4.0g of potassium carbonate into a 100mL round bottom flask, add 50mL of anhydrous acetonitrile, and react at 50°C for 5 hours. The reaction solution was lowered to room temperature, filtered, and the filtrate was concentrated and separated by silica gel column chromatography (ethyl acetate / petroleum ether=1 / 10) to obtain 3.63 g of white solid compound 7a with a yield of 69%. 1 H NMR (300MHz, CDCl 3 ): δ=7.41(dd,J=15.1,7.5Hz,3H),7.22(d,J=8.0Hz,2H),7.07(d,J=7.4Hz,1H),6.72(d,J=8.2Hz ,1H),5.31(s,2H),4.13(q,J=7.1Hz,2H),2.96(t,J=7.8Hz,2H),2.62(t,J=7.8Hz,2H),1.23(dd ,J=8.3,6.0Hz,3H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com