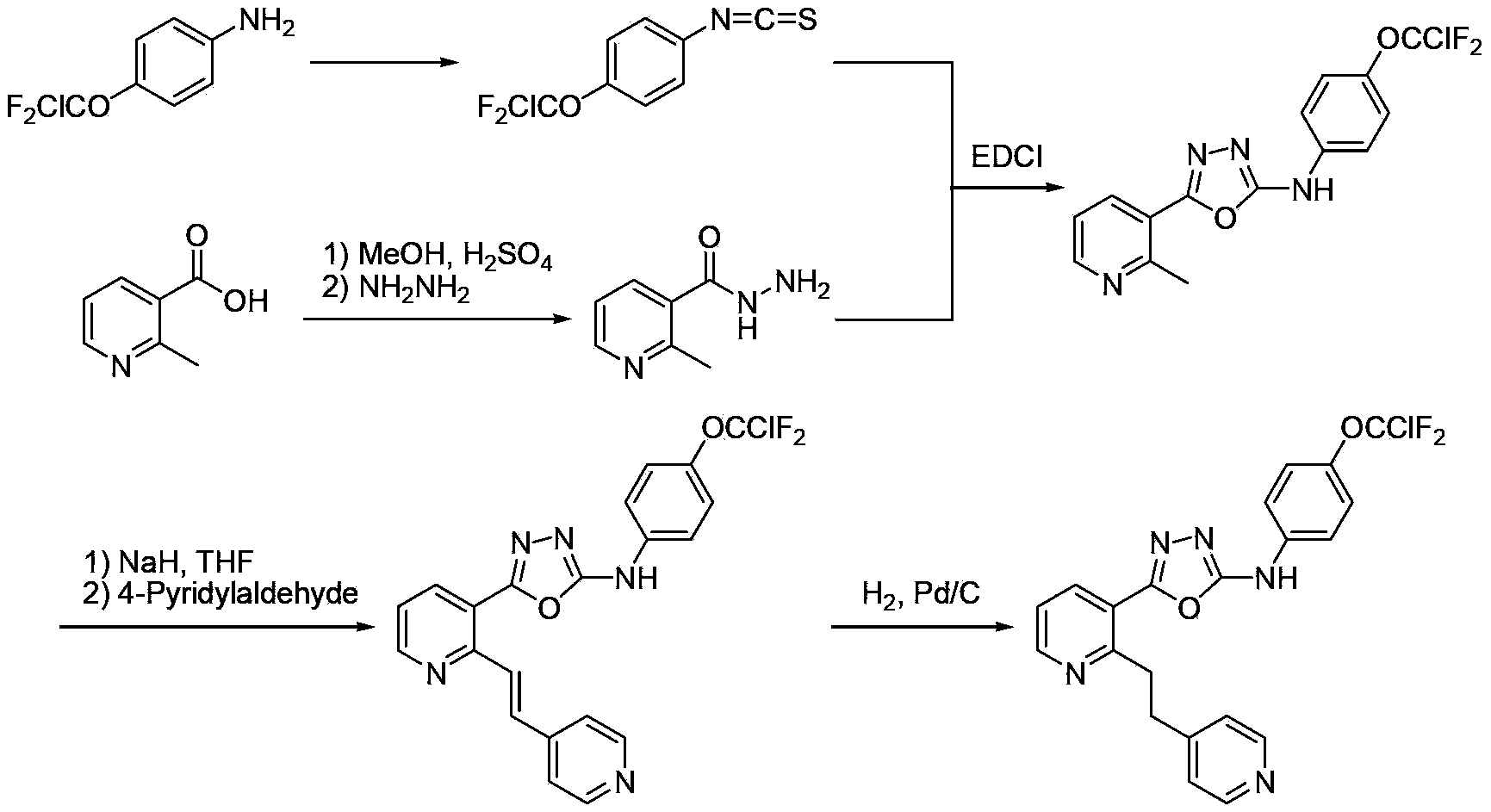
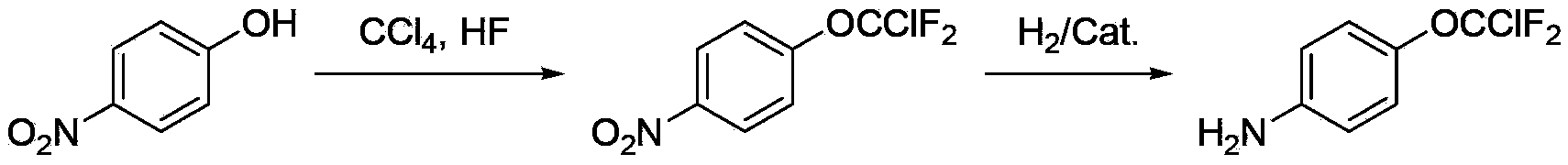
Preparation method for 4-(chlorodifluoromethoxy)aniline
A kind of technology of chlorodifluoromethoxybenzene and difluoromethoxy, which is applied in the field of preparation of 4-aniline and its intermediates, chlorodifluoromethoxybenzene and 4-nitrobenzene, and can solve the problem of industrial production Difficulty, use of dangerous sulfur phosgene, etc., to achieve the effect of less impurities, less nitric acid consumption, and clean process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] In a 1L stainless steel high-pressure reactor equipped with stirring and a thermometer, cool down to below 5°C, add 156g (7.8mol) of hydrogen fluoride, 156g (0.74mol) of trichloromethoxybenzene, and 0.9g of perfluorooctylsulfonyl Fluorine, heat up to 100-110°C, control the reaction pressure to 2.5-2.8MPa, and react for 4 hours. Sampling and GC detection showed that the content of 2-difluorochlorotrifluoromethoxybenzene was 81.5%. After the reaction is completed, excess hydrogen fluoride is removed by purging with nitrogen gas, and neutralized to pH=6-7 with potassium carbonate aqueous solution. Steam distillation followed by rectification gave 99 g of the product chlorodifluoromethoxybenzene with a yield of 74.9%.
Embodiment 2
[0029] In a 5L stainless steel high-pressure reactor equipped with stirring and a thermometer, cool down to below 5°C, add 780g (39mol) of hydrogen fluoride, 850g (4.02mol) of trichloromethoxybenzene, 5g of perfluorobutylsulfonyl fluoride, Raise the temperature to 100-110° C., control the reaction pressure to 2.5-2.8 MPa, and react for 4.5 hours. Sampling and GC detection showed that the content of chlorodifluorotrifluoromethoxybenzene was 79.8%. After the reaction is completed, excess hydrogen fluoride is removed by purging with nitrogen gas, and neutralized to pH=6-7 with potassium carbonate aqueous solution. Steam distillation followed by rectification to obtain 480 g of the product chlorodifluoromethoxybenzene with a yield of 72.6%.
Embodiment 3
[0031] In a 500ml three-necked flask equipped with a stirring, thermometer, and dropping funnel, add 313g (3.13mol) of 98% concentrated sulfuric acid, add 9g of water and 150g (0.84mol) of chlorodifluoromethoxybenzene, start stirring, and Add the prepared mixed acid (208g of sulfuric acid with a mass concentration of 98% and 56g of nitric acid with a mass concentration of 98%) dropwise at 15-20°C. After 1-2 hours, the dropwise addition is completed. After keeping for 2 hours, take a sample for analysis, and control the raw material ≤ 0.5% . After the analysis is qualified, the layers are static, and the spent acid is separated, and the organic phase is washed with 100 g of 5% sodium carbonate solution until neutral, and separated. The organic phase was washed with 50 g of water and separated to obtain 167 g of 4-nitrochlorodifluoromethoxybenzene, with a yield of 89%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com