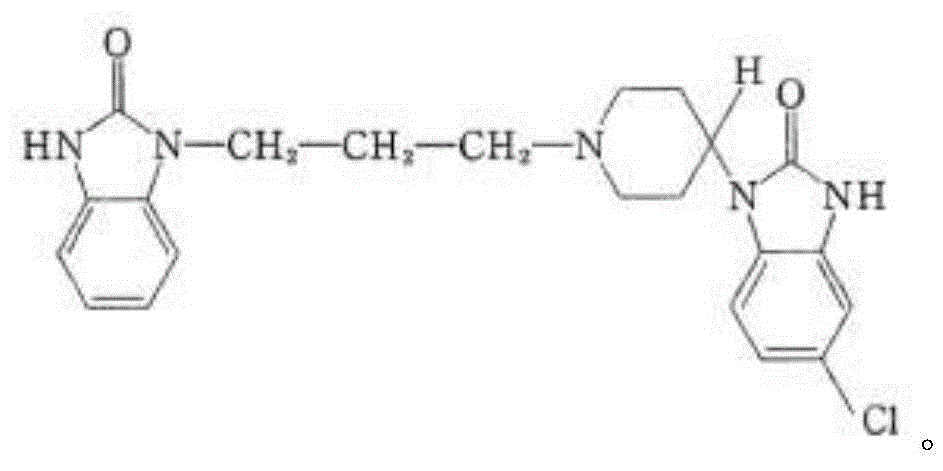
Domperidone tablet and preparation process thereof
A perperidone tablet and a technology for a preparation process, which are applied in the directions of medical preparations without active ingredients, medical preparations containing active ingredients, and pill delivery, etc., can solve the problem of long preparation cycle, simple preparation process, and increased gastrointestinal irritation. problems such as properties, to achieve the effect of easy industrialized large-scale production and simple preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035]
[0036] Preparation Process:
[0037] (1) Dissolve domperidone in 150 mL of ethanol, add lactose, disperse, stir evenly, and dry under reduced pressure at 50°C to remove ethanol. With the volatilization of ethanol, domperidone slowly precipitates and adsorbs on the surface of lactose to obtain a drug-containing adsorbate;
[0038] (2) Add the tert-butanol suspension of polacrilin potassium IRP-88 to the above-mentioned drug-containing adsorbate, stir evenly, remove tert-butanol by volatilization under reduced pressure at 70°C, and settle the drug-containing adsorbate of domperidone and lactose on On the surface of Polacrilin Potassium IRP-88, drug-containing solid deposits were obtained;
[0039] (3) Mix the drug-containing solid deposit with sodium stearate fumarate evenly, and press into tablets.
Embodiment 2
[0041]
[0042]
[0043] Preparation Process:
[0044] (1) Dissolve domperidone in 100 mL of ethanol, add lactose to disperse, stir evenly, and dry under reduced pressure at 55°C to remove ethanol. With the volatilization of ethanol, domperidone slowly precipitates and adsorbs on the surface of lactose to obtain a drug-containing adsorbate;
[0045] (2) Add the tert-butanol suspension of polacrilin potassium IRP-88 to the above-mentioned drug-containing adsorbate, stir evenly, evaporate the tert-butanol under reduced pressure at 74°C, and settle the drug-containing adsorbate of domperidone and lactose on On the surface of Polacrilin Potassium IRP-88, drug-containing solid deposits were obtained;
[0046] (3) Mix the drug-containing solid deposit with magnesium stearate evenly, and press into tablets.
Embodiment 3
[0048]
[0049] Preparation Process:
[0050] (1) Dissolve domperidone in 120 mL of ethanol, add lactose to disperse, stir evenly, and dry under reduced pressure at 50°C to remove ethanol. With the volatilization of ethanol, domperidone slowly precipitates and adsorbs on the surface of lactose to obtain a drug-containing adsorbate;
[0051] (2) Add the tert-butanol suspension of polacrilin potassium IRP-88 to the above-mentioned drug-containing adsorbate, stir evenly, remove tert-butanol by volatilization under reduced pressure at 70°C, and settle the drug-containing adsorbate of domperidone and lactose on On the surface of Polacrilin Potassium IRP-88, drug-containing solid deposits were obtained;
[0052] (3) Mix the drug-containing solid deposit with magnesium stearate evenly, and press into tablets.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com