CpG nucleic acid drug conveying system and making method thereof
A nucleic acid drug and delivery system technology, applied in the direction of drug combination, medical formula, genetic material components, etc., can solve the problems of uncontrollable release, immunogenic reaction, immune response, etc., to achieve increased storage capacity, improved inhibition and treatment, The effect of increasing secretion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
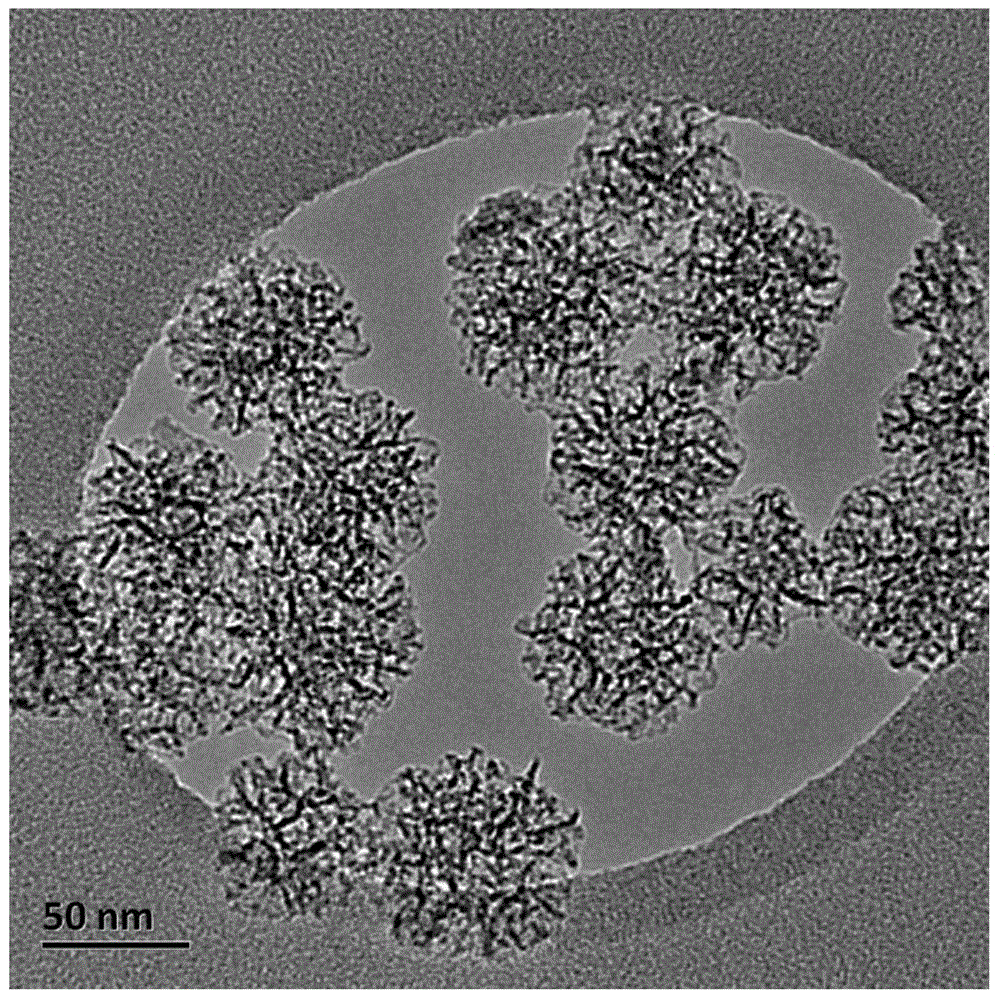
[0031] The CpG nucleic acid drug delivery system provided in this example includes a carrier and a CpG nucleic acid drug stored in the carrier, the carrier is mesoporous silica nanoparticles modified with an aminoalkane coupling agent, and the CpG nucleic acid drug is a CpG oligodeoxynucleotides, namely:
[0032] 5'-TCAGAGAGTTAGAGAGTTAGAGAGTCAGAGAGTTAGAGAGTTAGAGAGTCAGAGGTTAGAGAGTTAGAGAG-3'.
[0033] The aminosilane coupling agent used in the preparation method of the CpG nucleic acid drug delivery system is γ-aminopropyltriethoxysilane, the silicon source used is ethyl orthosilicate, and the surfactant used is cetyl p- Tosylate, the cosolvent that adopts is triethanolamine, specifically comprises following three steps:
[0034]Step 1: Dissolve 3eq of cetyl p-toluenesulfonate (CTAT) and 5eq of triethanolamine (TEA) in 4000eq of deionized water at 80°C, stir for 1 hour to dissolve completely; then slowly add After 18eq of tetraethyl orthosilicate (TEOS), continue to stir for 2...
Embodiment 2
[0045] The CpG nucleic acid drug delivery system provided in this example comprises a carrier and a CpG nucleic acid drug stored in the carrier, the carrier is an amino-modified mesoporous silica nanoparticle, and the CpG nucleic acid drug is a CpG oligodeoxynucleus containing 72 base pairs nucleotides, namely:
[0046] 5'-TCAGAGAGTTAGAGAGTTAGAGAGTCAGAGAGTTAGAGAGTTAGAGAGTCAGAGGTTAGAGAGTTAGAGAG-3'.
[0047] The aminosilane coupling agent used in the preparation method of the CpG nucleic acid drug delivery system is γ-aminopropyltriethoxysilane, the silicon source used is ethyl orthosilicate, and the surfactant used is cetyl p- Tosylate, the cosolvent that adopts is triethanolamine, specifically comprises following two steps:
[0048] Step 1: Dissolve 3eq of cetyl p-toluenesulfonate (CTAT) and 5eq of triethanolamine (TEA) in 4000eq of deionized water at 80°C, stir for 1 hour to dissolve completely; then slowly add After 16eq of tetraethyl orthosilicate (TEOS) and 2eq of γ-amin...
Embodiment 3
[0053] The CpG nucleic acid drug used in this example is the CpG oligodeoxynucleotide used in Example 1, and the carrier used is the carrier prepared in Example 1.
[0054] The carrier prepared in Example 1 was dispersed in deionized water to prepare a 1 μg / μl suspension. The suspension and the CpG nucleic acid drug were mixed at a mass ratio of 5:1 to prepare a suspension of the two, then shaken in a shaker at room temperature for 4 hours, centrifuged, and washed with deionized water to obtain a CpG nucleic acid drug storage The amino-modified mesoporous silica nanoparticles are the target CpG nucleic acid drug delivery system.
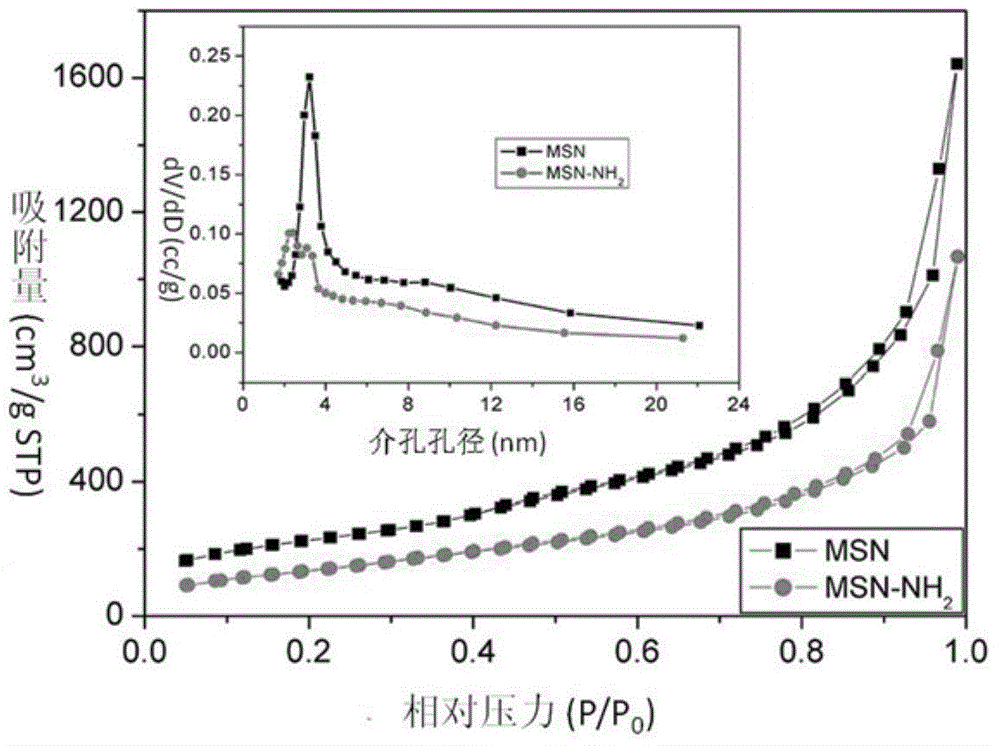
[0055] The obtained CpG nucleic acid drug delivery system was measured by an ultramicro spectrophotometer, wherein the storage capacity of the CpG nucleic acid drug was 116 μg / mg.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Mesopore diameter | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com