Method for removing iron from titanium dioxide concentrated acid solution
A technology for concentrating acid and titanium dioxide, which is applied in the direction of iron sulfate, phosphoric acid, phosphorus oxyacid, etc., can solve the problems of unfavorable production of high-quality ammonium phosphate products, failure to increase the water solubility of ammonium phosphate, and failure to reduce iron content, etc. Achieve the effects of small investment, low production cost, and reduced iron content
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
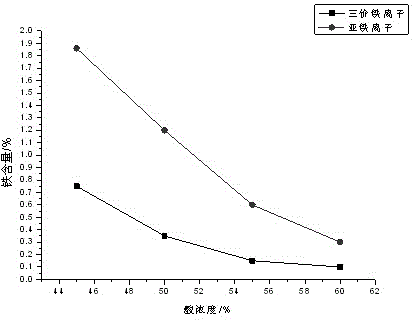
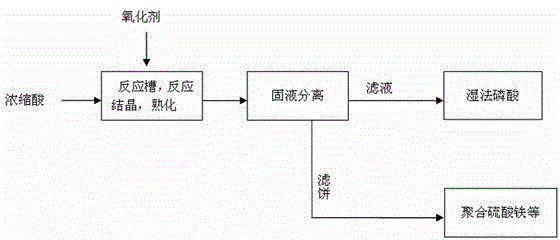
[0021] Put 1000kg of concentrated titanium dioxide acid (54% by mass of sulfuric acid, 0.48% by mass of ferrous iron, and a temperature of 55°C) and 5.6kg of industrial hydrogen peroxide (27.5% by mass of hydrogen peroxide) through the pipeline Enter 1m 3 Add 3kg of ferric sulfate as a seed crystal in the reaction tank, oxidize and crystallize under stirring conditions, the stirring speed is 100rpm, and the crystallization time is 3h. After the crystallized slurry is filtered by a filter press, the filtrate is sent to wet-process phosphoric acid use.
[0022] In the present embodiment, the concentrated acid iron content is reduced to 0.15%, and the iron removal rate is 69%.
Embodiment 2
[0024] Pass 1000kg of concentrated titanium dioxide acid (48% by mass of sulfuric acid, 0.55% by mass of ferrous iron, and 55°C) and 6.5kg of industrial hydrogen peroxide (27.5% by mass of hydrogen peroxide) through the pipeline Enter 1m 3 Add 3kg of ferric sulfate as a seed crystal in the reaction tank, oxidize and crystallize under stirring conditions, the stirring speed is 100rpm, and the crystallization time is 2.5h. After the crystallized slurry is filtered by a filter press, the filtrate is sent to the wet process phosphoric acid used.
[0025] In the present embodiment, the concentrated acid iron content is reduced to 0.19%, and the iron removal rate is 65%.
Embodiment 3
[0027] Put 1000kg of concentrated titanium dioxide acid (54% by mass of sulfuric acid, 0.48% by mass of ferrous iron, and a temperature of 55°C) and 5.6kg of industrial hydrogen peroxide (27.5% by mass of hydrogen peroxide) through the pipeline Enter 1m 3 Add 5kg of ferric sulfate as a seed crystal in the reaction tank, oxidize and crystallize under stirring conditions, the stirring speed is 100rpm, and the crystallization time is 3h. For 0.5h, the supernatant is sent to wet-process phosphoric acid for use, the lower layer of thick slurry is filtered with a small filter press, and the filtrate is sent to wet-process phosphoric acid.
[0028] In this implementation case, the content of concentrated acid iron is reduced to 0.14%, and the iron removal rate is 70%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com