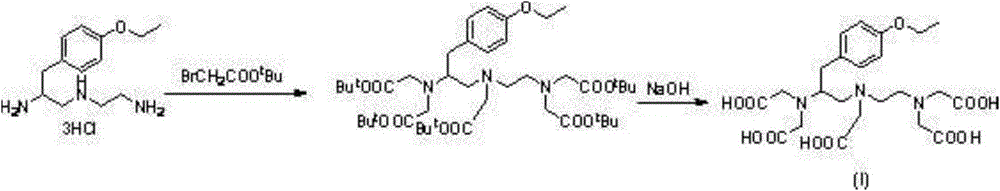
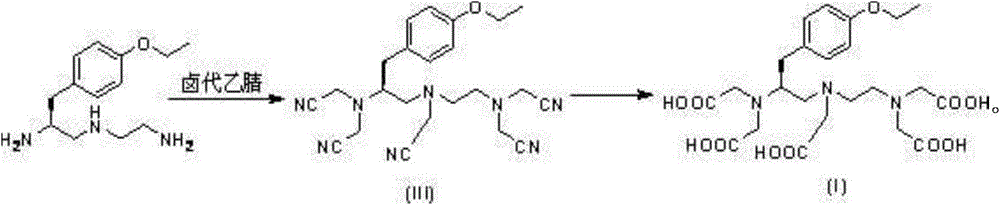
Preparation method of (4S)-3, 6, 9-triaza-3, 6, 9-tri(carboxymethyl)-4-(4-ethoxy benzyl)undecanedioic acid
A technology of ethoxybenzyl and triaza, which is applied in the field of -3, can solve the problems of being unsuitable for industrial production, achieve the effect of being suitable for industrial production and avoiding chromatographic steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Synthesis of (4S)-3,6,9-triaza-3,6,9-tris(nitrilemethyl)-4-(4-ethoxybenzyl)undecanedinitrile
[0026] Add (4S)-1-(4-ethoxybenzyl)-3-azopentane-1,5-diamine trihydrochloride (34.6g, 0.1moL, 1.0eq. ), THF346mL, water (70mL, 2P) and potassium carbonate (110.4g, 0.8moL, 8.0eq.). After the solution was stirred and clarified, bromoacetonitrile (89.96g, 0.75moL, 7.5eq.) was added dropwise at room temperature. After the dropwise addition, the temperature was raised to 80°C and refluxed for 6 hours. Extract 300mL×2, collect the organic layer, wash 200mL×1 with saturated brine, and dry over anhydrous sodium sulfate. Filtered, evaporated to dryness with a water pump, and recrystallized from ethyl acetate to obtain (4S)-3,6,9-triaza-3,6,9-tris(nitrilemethyl)-4-(4-ethane) as a light yellow solid Oxybenzyl)undecanedinitrile (26 g, 60% yield).
[0027] 1 H NMR (400MHz, CDCl 3 ): δ=1.31-1.35 (t, 3H, CH 3 ), 2.30-2.73 (m, 8H, 4CH 2 ), 3.25(m, 1H, CH), 3.48(s, 10H, 5CH 2 ), 3.95-4...
Embodiment 2
[0029] Synthesis of (4S)-3,6,9-triaza-3,6,9-tris(nitrilemethyl)-4-(4-ethoxybenzyl)undecanedinitrile
[0030] Add (4S)-1-(4-ethoxybenzyl)-3-azopentane-1,5-diamine (34.6g, 0.1moL, 1.0eq.), THF346mL to a 1L three-necked flask, Water 70 mL, and sodium carbonate (110 g, 0.8 moL, 8.0 eq.). After the solution was stirred and clarified, chloroacetonitrile (56.2g, 0.75moL, 7.5eq.) was added dropwise at room temperature. After the dropwise addition, the temperature was raised to 80°C and refluxed for 5 hours. Extract 300mL×2, collect the organic layer, wash 200mL×1 with saturated brine, and dry over anhydrous sodium sulfate. Filtered, evaporated to dryness with a water pump, and recrystallized from ethyl acetate to obtain (4S)-3,6,9-triaza-3,6,9-tris(nitrilemethyl)-4-(4-ethane) as a light yellow solid Oxybenzyl)undecanedinitrile (25 g, 57% yield).
[0031] 1 H NMR (400MHz, CDCl 3 ): δ=1.31-1.35 (t, 3H, CH 3 ), 2.302.73 (m, 8H, 4CH 2 ), 3.25(m, 1H, CH), 3.48(s, 10H, 5CH 2 ), 3.95...
Embodiment 3
[0033]Synthesis of (4S)-3,6,9-triaza-3,6,9-tris(carboxymethyl)-4-(4-ethoxybenzyl)undecanedioic acid -3,6,9-Triaza-3,6,9-tris(nitrilemethyl)-4-(4-ethoxybenzyl)undecanedinitrile (26g, 0.06moL, 1.0eq.) Add methanol (390mL, 15P) to a 1L three-neck flask to dissolve, then add sodium hydroxide (60g, 1.5moL, 25eq.) and water (13mL, 0.5P) solution, and heat up and reflux for 24 hours. After the reaction, concentrate the reaction solution, beat with ethanol to remove excess sodium hydroxide, then dissolve it in 80mL of water, adjust the pH value of the solution to 2.0 with 1N HCl solution under ice bath, filter, wash with water, dry with hot water (100°C) recrystallization to give white solid (4S)-3,6,9-triaza-3,6,9-tris(carboxymethyl)-4-(4-ethoxybenzyl)undecane Diacid (19 g, 60% yield).
[0034] 1 H NMR (400MHz, DMSO): = 1.31-1.35(t, 3H, CH 3 ), 2.34-2.83 (m, 8H, 4CH 2 ), 3.24(m, 1H, CH), 3.12-3.37(s, 10H, 5CH 2 ), 3.91-4.01 (m, 2H, CH 2 ), 6.79-6.81 (d, 2H, J=8.0Hz, ArH), 7.09...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com