Method for production of 5-hydroxymethylfurfural or levulinic acid from inulin biomass
A technology of hydroxymethyl furfural and levulinic acid, applied in chemical instruments and methods, preparation of organic compounds, preparation of carboxylates, etc., can solve problems such as high energy consumption, difficult control of product 5-HMF or levulinic acid, etc. , to achieve the effect of low reaction temperature, short reaction time and high substrate concentration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
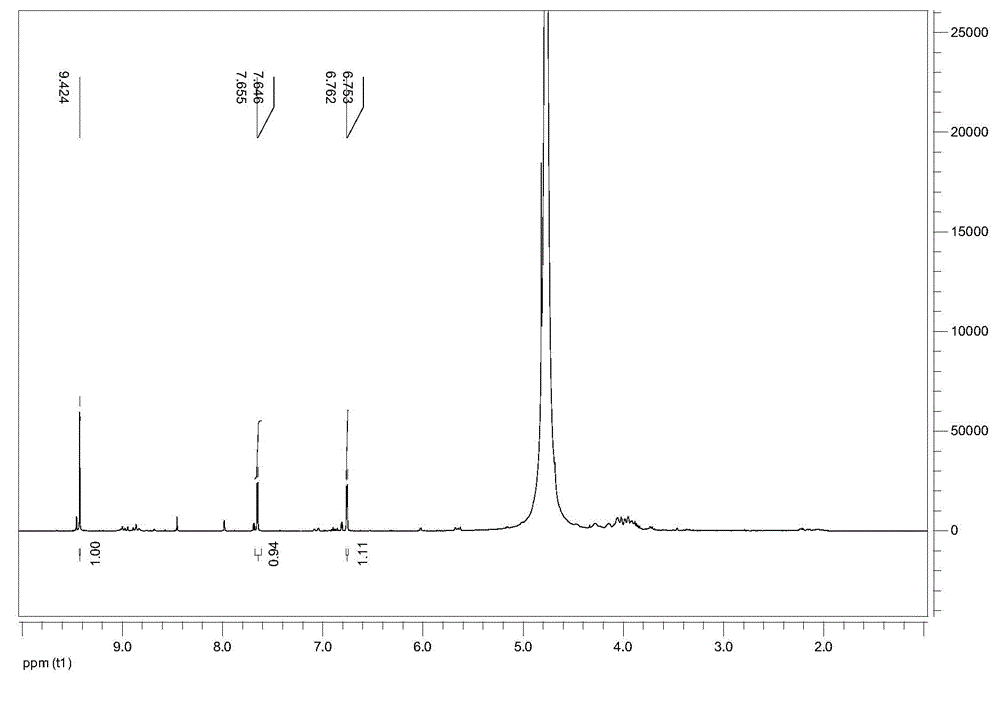
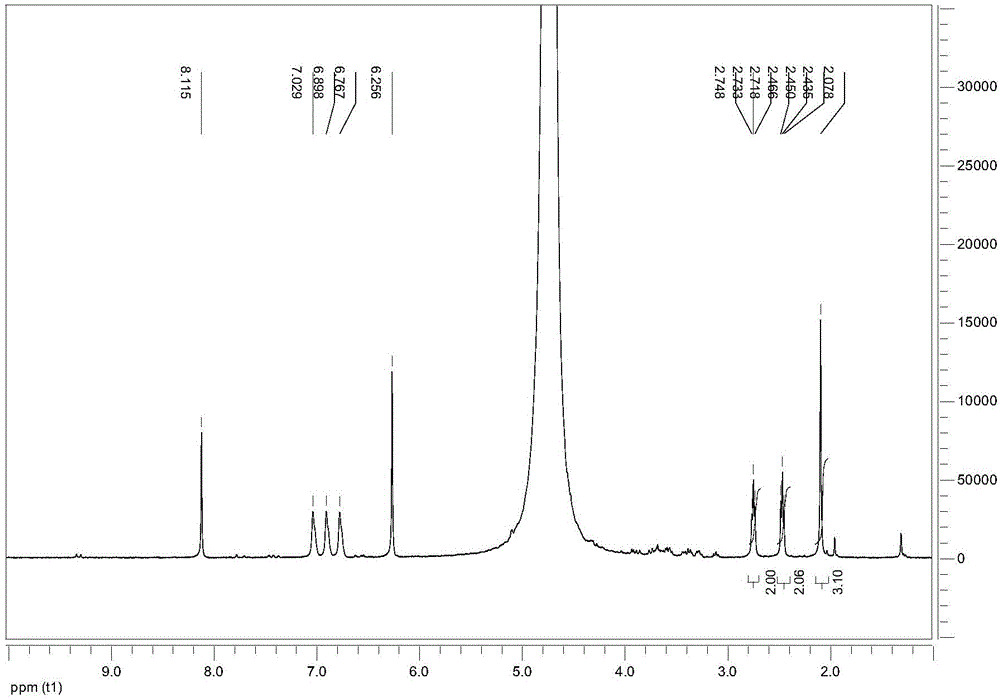
Image
Examples
Embodiment 1
[0023] (1) Prepare 30g of 32% zinc chloride aqueous solution by mass, add 5g of fructose, and react at 120°C for 2 hours.
[0024] (2) After the reaction, 75 mL of butanol was added as an extractant to extract the product 5-HMF.
[0025] (3) Concentrate and evaporate to dryness the obtained butanol phase in which the product 5-HMF is dissolved, and crystallize the obtained solid in petroleum ether-ethyl acetate (1:2 volume ratio) to obtain the 5-HMF product, 5-HMF The molar yield is 35%.
Embodiment 2
[0027] (1) Prepare 50g of zinc chloride aqueous solution with a mass percentage of 67%, add 10g of sucrose with a molecular weight of 342 to form a reaction solution, and react at 100°C for 15 minutes.
[0028] (2) After the reaction, add 120mL methyl isobutyl ketone as an extractant to extract 5-HMF.
[0029] (3) The obtained methyl isobutyl ketone phase containing the product 5-HMF was concentrated and evaporated to dryness, and the obtained solid was crystallized with benzene to obtain the 5-HMF product, and the molar yield of the 5-HMF product was 43%.
Embodiment 3
[0031] (1) Prepare 30g of zinc chloride aqueous solution with a mass percentage of 60%, add 10g of inulin with a molecular weight of 20,000 to mix, add 0.2g of SnCl 4 ·5H 2 O was used as a catalyst, and the reaction was continued at 140 °C for 8 hours.
[0032] (2) After the reaction, add 70mL of 2,5-dimethylpentanone as an extractant to extract the product levulinic acid.
[0033] (3) Concentrate the obtained 2,5-dimethylpentanone by rotary evaporation under reduced pressure, crystallize with a mixed solvent of 1,4-dioxane-ether to obtain the levulinic acid product, and the molar yield of the levulinic acid product is The rate is 55%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com