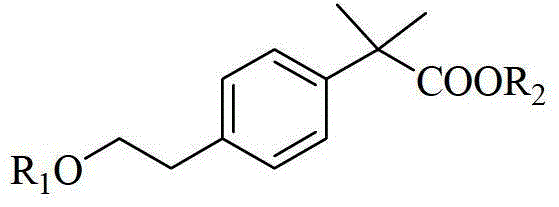
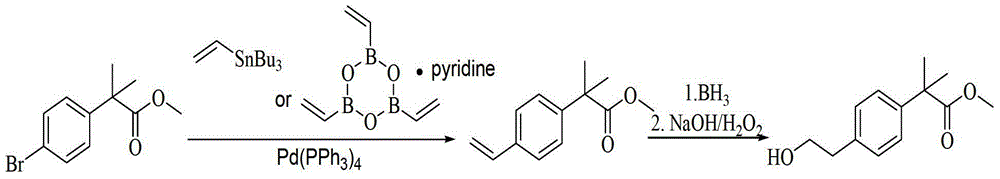
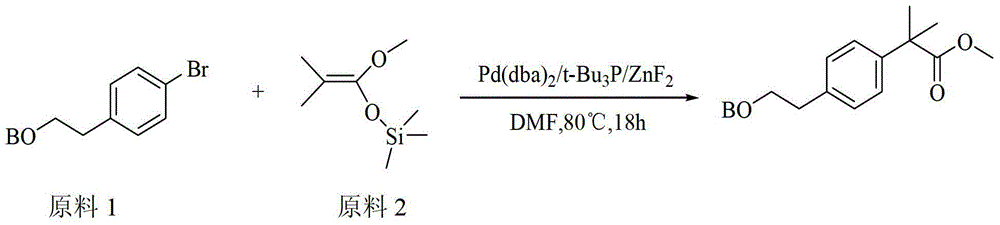Method for synthesizing 2-methacrylate derivatives by use of ester compounds
The technology of a compound and a palladium compound is applied in the field of synthesis of pharmaceutical intermediates, and can solve the problems of difficulty in purchasing raw material tin compounds, large pollution, etc., and achieve the effects of being beneficial to large-scale production, reducing energy consumption, and easily obtaining raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 12-4
[0055] The synthesis of embodiment 12-[4-[2-(ethoxy) ethyl] phenyl]-2-methoxybenzyl propionate
[0056] Add the toluene solution containing 14.14g lithium bistrimethylsilylamide to the reaction flask, then add 70.37g p-methoxybenzyl isobutyrate (formula III, R2 is p-methoxybenzyl), after stirring for 10 minutes, Add 10.4g4-[2-(ethoxy)ethyl]chlorobenzene (formula II, R1 is ethyl, X is chlorine) and 4.5mg dibromobis(tri-tert-butylphosphine)palladium(I), in After reacting for 40 minutes at 20-30 degrees, the raw material 4-[2-(ethoxy)ethyl]chlorobenzene was detected by HPLC method and disappeared. After the reaction is complete, add 1mol / L hydrochloric acid to the reaction solution to precipitate a solid, filter and retain the filtrate, separate the organic layer from the filtrate, and concentrate to obtain 2-[4-[2-(ethoxy)ethyl]phenyl] - 15.85 g of p-methoxybenzyl 2-methylpropionate, the molar yield is 91.2%.
Embodiment 22-4
[0057] The synthesis of embodiment 22-[4-[2-(cyclohexyloxy) ethyl] phenyl]-2-methyl propionate
[0058] Add 3.88g of tetrahydrofuran solution containing lithium diisopropylamide to the reaction flask, then add 3.70g of methyl isobutyrate (formula III, R2 is methyl), stir for 12 minutes, then add 20.5g of 4-[2 -(cyclohexyloxy) ethyl] bromobenzene (formula II, R1 is cyclohexyl, X is bromine) and 0.8365g tetrakis (triphenylphosphine) palladium (0), reacted for 60 minutes at 15-25 degrees, The HPLC method detected that the raw material 4-[2-(cyclohexyloxy)ethyl]bromobenzene had disappeared. After the reaction is complete, add 1mol / L hydrochloric acid to the reaction solution to precipitate a solid, filter and retain the filtrate, separate the organic layer from the filtrate, and concentrate to obtain 2-[4-[2-(cyclohexyloxy)ethyl]phenyl ]-21.51g of methyl propionate, molar yield 97.6%.
Embodiment 32-4
[0059] Synthesis of Example 32-[4-[2-(benzyloxy)ethyl]phenyl]-2-methylpropionic acid isopropyl ester
[0060]Add the ethylene glycol dimethyl ether solution containing 55.30g of lithium dicyclohexylamide to the reaction flask, then add 7.69g of isopropyl isobutyrate (formula III, R2 is isopropyl), and stir for 8 minutes. Add 8.6g 4-[2-(benzyloxy)ethyl]bromobenzene (formula II, R1 is benzyl, X is bromine) and 34.1mg tetrakis(triphenylphosphine) palladium (0), at 0-10 degrees The reaction was carried out for 110 minutes, and the raw material 4-[2-(benzyloxy)ethyl]bromobenzene had disappeared as detected by HPLC. After the reaction is complete, add 1mol / L hydrochloric acid to the reaction solution to precipitate a solid, filter and retain the filtrate, separate the organic layer from the filtrate, and concentrate to obtain 2-[4-[2-(benzyloxy)ethyl]phenyl] -9.70 g of isopropyl 2-methylpropionate, the molar yield is 96.5%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com