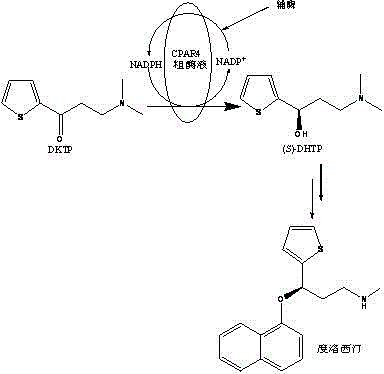
A method for synthesizing (s)-n,n-dimethyl-3-hydroxyl-3-(2-thiophene)-1-propanamine catalyzed by the recombinant bacterial crude enzyme system of aldehyde and ketone reductase
A technology of reductase and recombinant bacteria, which is applied in the field of biocatalytic asymmetric transformation, can solve the problems of low level, limited industrial application, and affecting the space-time yield of catalytic reactions, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] The preparation of embodiment 1 recombinant bacteria crude enzyme system:
[0044] The composition of LB medium was tryptone 1%, yeast extract 0.5%, NaCl 1%, pH 7.0. When needed, add ampicillin (50 μg / mL) before use, and add 1.5% agar powder to the solid medium.
[0045] A single colony of the positive clone was picked and inoculated in 3 mL LB liquid medium containing 50 μg / mL ampicillin, and cultured overnight at 37 °C with shaking at 200 rpm. Take 1 mL of the culture solution and transfer it to 50 mL of LB liquid medium containing 50 μg / mL ampicillin, and culture at 37°C and 200 rpm until OD 600 About 0.6. The inducer IPTG was added to the culture to a final concentration of 1 mmol / L, and induced at a culture temperature of 17°C for 12 h.
[0046] The cultured recombinant E. coli cells were collected by centrifugation at 10,000 rpm for 10 min and washed three times with saline. The bacteria were resuspended in potassium phosphate buffer (pH 6.5, 0.1 M) to form a ...
Embodiment 2
[0047] The crude enzyme system catalyzes the asymmetric reduction reaction in the aqueous phase reaction system of embodiment 2:
[0048] 2 mL reaction system consists of 1 mL potassium phosphate buffer (pH 6.5, 0.1 M), 1 mL crude enzyme system (total soluble protein 15 mg), 10 g / L glucose, 5 g / L DKTP, 0.02 mM NADP + . The reaction mixture was shaken at 30° C. for 8 h, and the reaction mixture was extracted with 2 times the volume of ethyl acetate, and the product ( S )-DHTP has an optical purity of 99% e.e. and a yield of 69.4%.
Embodiment 3
[0049] The crude enzyme system catalyzes the asymmetric reduction reaction in the aqueous phase reaction system of embodiment 3:
[0050] The 2 mL reaction system consists of 1 mL potassium phosphate buffer (pH 6.5, 0.1 M), 1 mL crude enzyme system (total soluble protein 15 mg), 10 g / L lactose, 5 g / L DKTP, 0.02 mM NADP + . The reaction mixture was shaken at 30° C. for 8 h, and the reaction mixture was extracted with 2 times the volume of ethyl acetate, and the product ( S )-DHTP has an optical purity of 99% e.e. and a yield of 69.7%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com