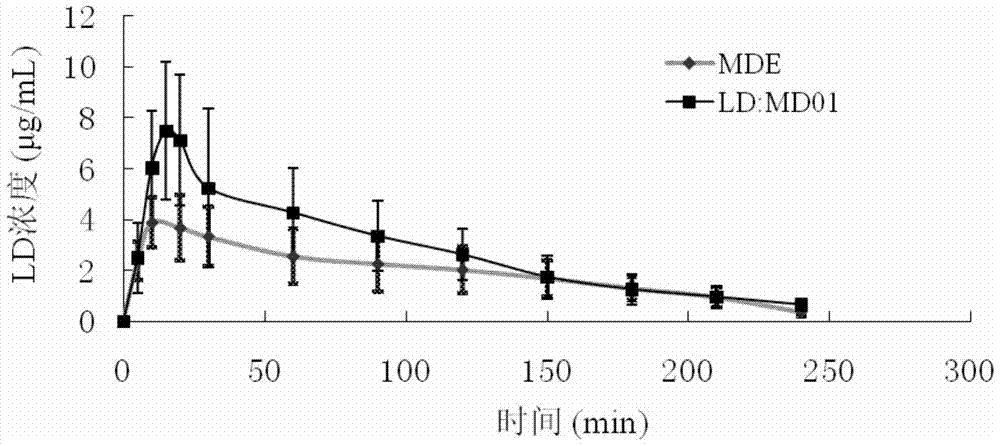
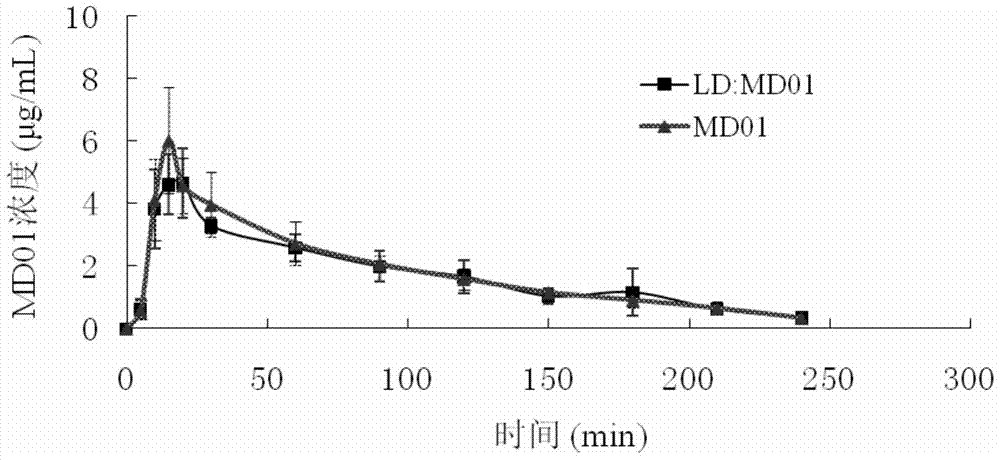
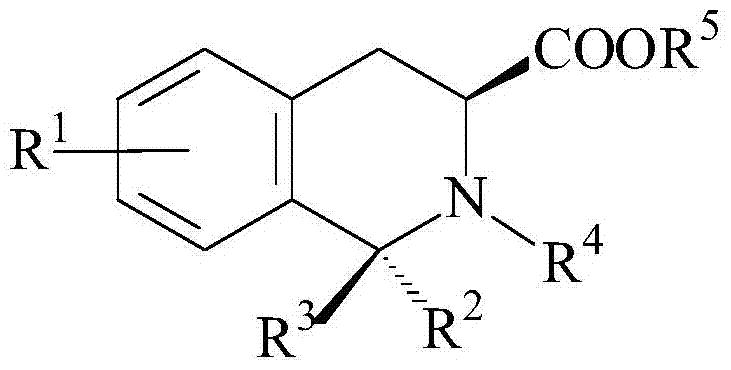
Application of 3-carboxylic acid tetrahydroisoquinoline derivatives in the preparation of drugs for treating dopaminergic neurological diseases
A tetrahydroisoquinoline, dopaminergic technology, applied in the field of biomedicine, can solve problems such as rare pharmacological activity, and achieve the effects of enhancing and brain bioavailability and reducing the rate of LD degradation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Preparation of 3-Carboxylic Acid Tetrahydroisoquinoline Derivatives from Cat Bean
[0022] The medicinal material of cat bean (MD20091101) was collected in Baise, Guangxi, and was identified as the seed of Mucuna pruriens var. utilis A. Cheval. Take 3.0 kg of dried cat bean, grind it into coarse powder, extract it with acetone at room temperature for 24 hours to remove the oil, and continue to extract the dregs with methanol for 2 times under reflux, each time for 2 hours, and recover methanol to obtain 214 g of cat bean extract (MDE). Take MDE104g, after repeated medium-pressure silica gel column chromatography, elute with chloroform-methanol-0.5% acetic acid 7:3:0.5 (volume ratio) to obtain MD02 (30mg); continue to elute with 6.5:3.5:0.5 to obtain MD01 (200 mg), MD03 (12 mg); continue to elute at 5:5:0.5, and obtain MD04 (15 mg), LD (35 mg); in sequence. The chemical structures of MD01-MD04 have been 1 H-NMR, IR, MS and other spectral analysis, compared with the lit...
Embodiment 2
[0024] Chemical Synthesis of (3S)-3-carboxy-6,7-dihydroxy-1,2,3,4-tetrahydroisoquinoline (MD03)
[0025] Take 100mg of levodopa, add appropriate amount of 0.1N hydrochloric acid to dissolve, neutralize with sodium hydroxide solution, add 0.35ml of 40% formaldehyde solution, shake and mix, stir and react at 30°C for 72h. The reaction solution was centrifuged (4000r / min, 10min), and the precipitate was collected and dried under reduced pressure to obtain 20 mg of the target product.
Embodiment 3
[0027] Chemical Synthesis of 1-methyl-3-carboxy-6,7-dihydroxy-1,2,3,4-tetrahydroisoquinoline
[0028]Take 100mg of levodopa, add appropriate amount of 0.1N hydrochloric acid to dissolve, neutralize with sodium hydroxide solution, add 0.35ml of acetaldehyde solution, shake and mix, stir and react at 30°C for 72h. The reaction solution was centrifuged (4000r / min, 10min), and the precipitate was collected and dried under reduced pressure to obtain 50mg of the product. The product was further separated by HPLC. The chromatographic conditions were: ODS chromatographic column, 20×250mm, 10% methanol-0.1% acetic acid as the mobile phase, to obtain 1α-methyl-3-carboxy-6,7-dihydroxy-1 , 15 mg of 2,3,4-tetrahydroisoquinoline, 8 mg of 1β-methyl-3-carboxy-6,7-dihydroxy-1,2,3,4-tetrahydroisoquinoline.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com