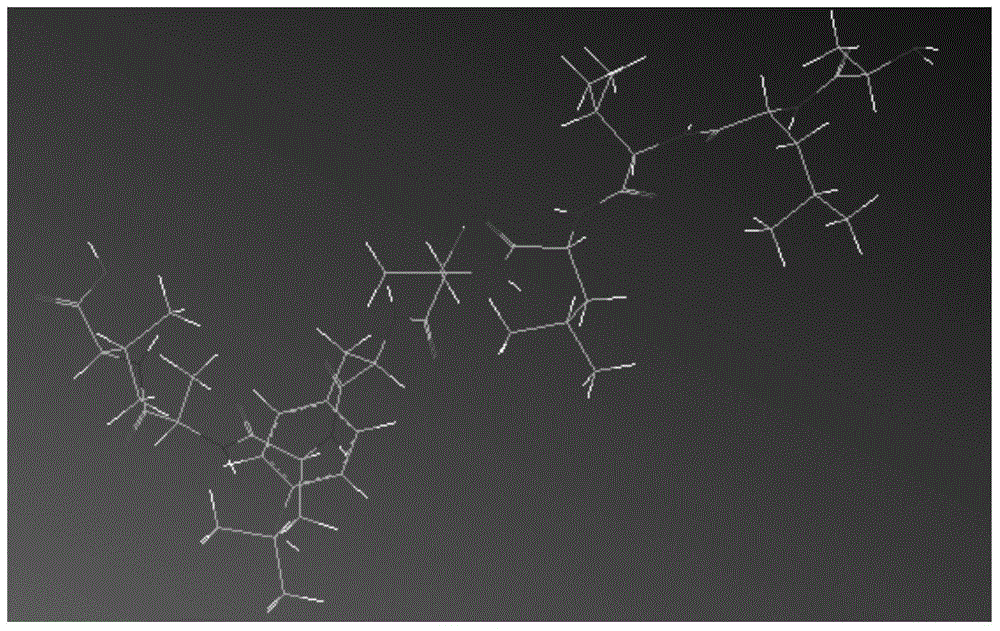
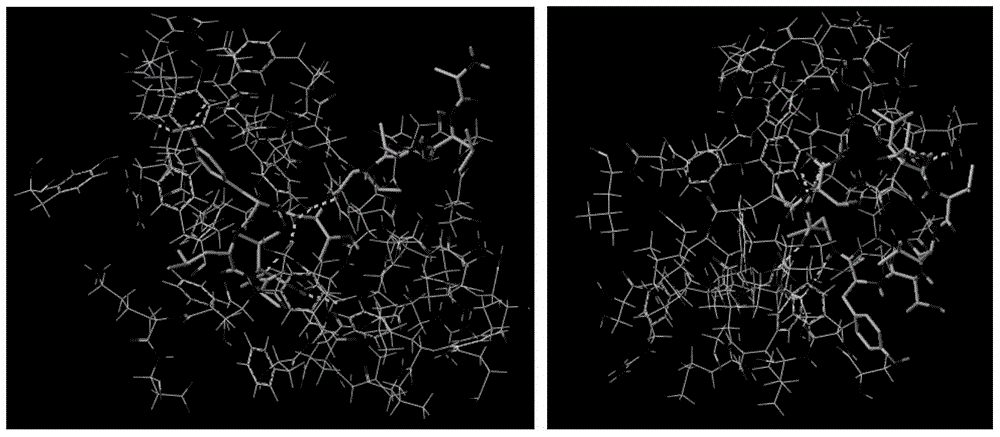
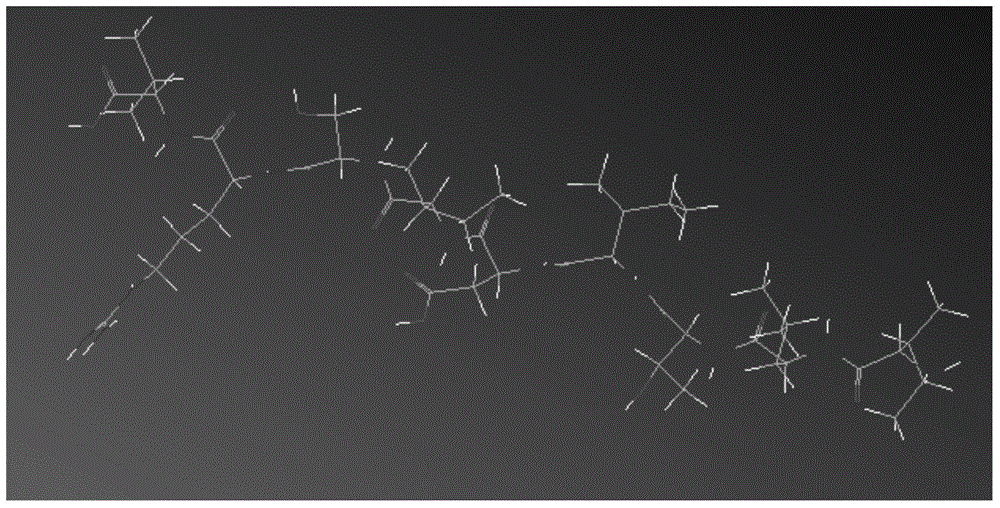
ECM1 polypeptide epitopes bound to human MHC-class I molecules
A MHC-I, -1ECM1 technology, applied in the direction of medical preparations containing active ingredients, peptides, analytical materials, etc., to achieve the effect of large commercial industrialization, convenient clinical application, and improved accuracy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Example 1 Screening method for polypeptide epitopes
[0031] The polypeptide sequence obtained in the present invention is directly retrieved from the peptide library through the combination of the supermotif method and the quantitative motif method, and maintains the binding property of MHC-I molecules. Through probabilistic and statistical analysis of these polypeptide sequences, a binding model of the polypeptide and MHC-I class molecules is obtained.
[0032] 1. Obtaining the amino acid sequence of human ECM1
[0033] Find the full-length amino group of natural human ECM1 from the international open shared gene bank NCBI GeneBank
[0034] The acid sequence (540 amino acids in total) is represented as:
[0035] MGTTARAALV LTYLAVASAA SEGGFTATGQ RQLRPEHFQE VGYAAPPSPP
[0036] LSRSLPMDHP DSSQHGPPFE GQSQVQPPPS QEATPLQQEK LLPAQLPAEK
[0037] EVGPPLPQEA VPLQKELPSL QHPNEQKEGM PAPFGDQSHP EPESWNAAQH
[0038] CQQDRSQGGW GHRLDGFPPG RPSPDNLNQI CLPNRQHVVY GPWNLPQSSY
[0039...
Embodiment 2
[0065] Example 2 Computer Simulation of Polypeptide Epitopes Binding to MHC-I Class Molecules
[0066] Use ChemDraw Ultra and ChemDraw3D Ultra in the ChemOffice software package to establish the two-dimensional and three-dimensional structure of the polypeptide epitope obtained above; use SYBYL-X software to analyze the energy and structure of the polypeptide epitope and HLA-A2.1 Modification, simulating the three-dimensional structure of the combination of the two, carrying out molecular dynamics combination simulation and estimation of physiological activity and application value, mainly include the following methods: (1) Construction of polypeptide epitope molecular model. Use ChemDraw Ultra in the ChemOffice software package to construct the two-dimensional structure of the peptide epitope molecule, then import the two-dimensional structure of the peptide epitope into ChemDraw3D Ultra to obtain the three-dimensional model and save it in Mol2 format, and import the three-dim...
Embodiment 3
[0067] Example 3 Synthesis, purification and molecular weight determination of polypeptide epitopes
[0068] The peptide epitope was synthesized by Jill Biochemical (Shanghai) Co., Ltd. using the standard Fmoc protocol, and the purity was detected by high performance liquid chromatography (HPLC), and the molecular weight was determined by mass spectrometry. For its mass spectrogram, see Figure 5 and Figure 7 , it can be seen that the theoretical values of the two polypeptides are close to the measured values, within the allowable error range. HPLC analysis chart see Figure 6 and Figure 8 , it can be seen that the purity is above 95%, indicating that the synthesis effect is good. The above peptides were freeze-dried and stored at -70°C for future use.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com