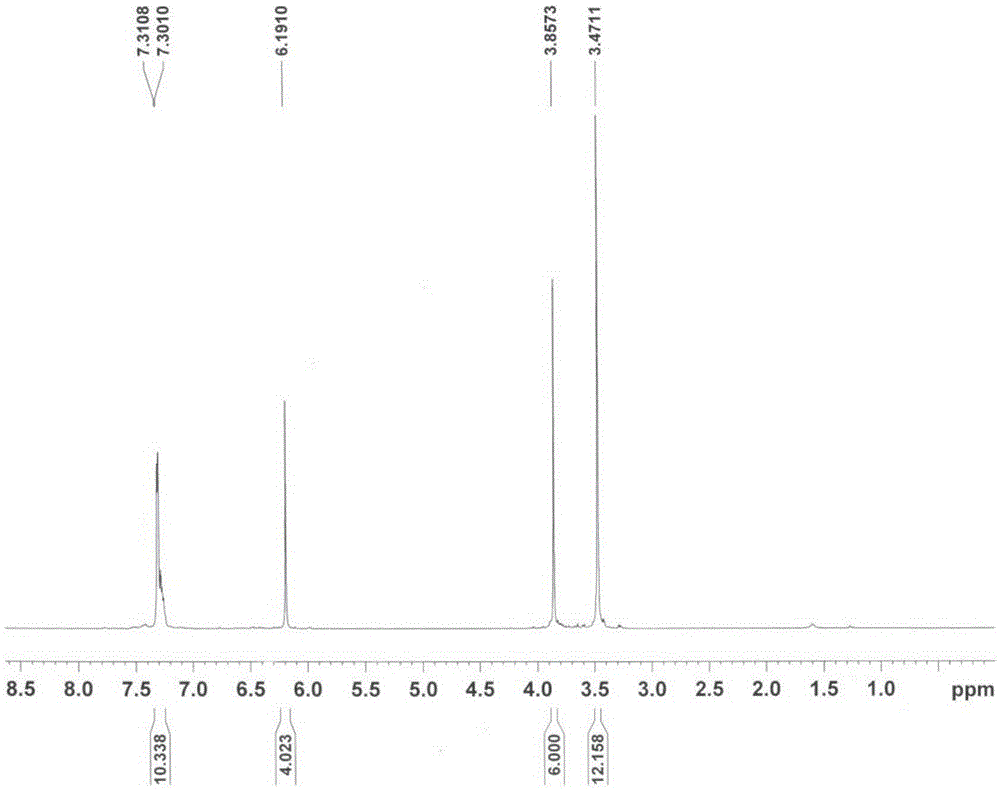
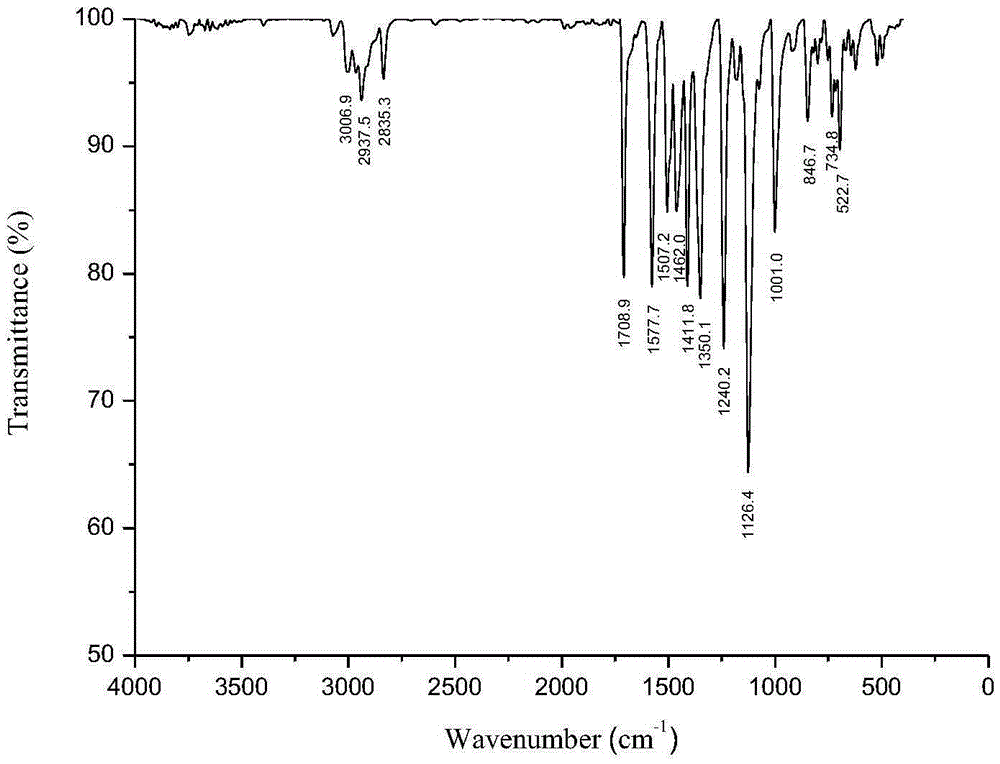
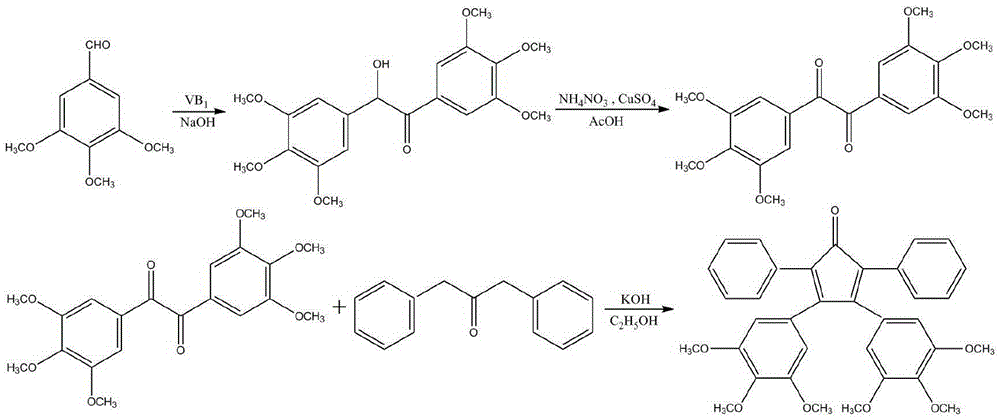
Method for synthesizing 3,4-bis(3,4,5-trimethoxyphenyl)-2,5-diphenylcyclopentadienone
A technology of diphenylcyclopentadienone and trimethoxyphenyl, which is applied in the field of organic compound synthesis, can solve the problems of high price of reaction raw materials, cumbersome and time-consuming steps, and limited application, so as to achieve safe and reliable reaction process and comprehensive synthesis. Simple process and good reproducibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] (1) Preparation of 4,4'-bis(3,4,5-trimethoxy)benzoin
[0035] Add vitamin B to the three-neck round bottom flask equipped with reflux condensation, stirring device and thermometer in sequence 1 2g, 5mL of distilled water, 16mL of ethanol, and 15.3g of 3,4,5-trimethoxybenzaldehyde, and slowly stirred in an ice-water bath for 15min. Then add 3.0 mol / L sodium hydroxide ethanol solution dropwise through the condenser to adjust the pH value of the solution to 8, and continuously add 3.0 mol / L sodium hydroxide ethanol solution to keep the pH value of the solution for 45 minutes. After heating to 55°C for 3h, cool to room temperature. After standing for 24 hours, suction filtration, the filter cake was washed 3 times with distilled water, and the filtrate was neutral to obtain a yellow solid. After recrystallization with ethanol, 9.2g of 4,4'-bis(3,4,5-trimethoxy)benzoin was obtained, the yield was 60.2%, m.p: 165.2-165.6°C (literature value (WO2010050720A2): 165-166 ℃).
...
Embodiment 2
[0045] (1) Preparation of 4,4'-bis(3,4,5-trimethoxy)benzoin
[0046] Add vitamin B to the three-neck round bottom flask equipped with reflux condensation, stirring device and thermometer in sequence 1 4g, 10mL of distilled water, 32mL of ethanol, 30.6g of 3,4,5-trimethoxybenzaldehyde, and slowly stir in an ice-water bath for 15min. Then add 3.0 mol / L sodium hydroxide ethanol solution dropwise through the condenser to adjust the pH value of the solution to 8, and continuously add 3.0 mol / L sodium hydroxide ethanol solution to keep the pH value of the solution for 45 minutes. After heating to 65°C for 3h, cool to room temperature. After standing for 24 hours, suction filtration, the filter cake was washed 3 times with distilled water, and the filtrate was neutral to obtain a yellow solid. After recrystallization from ethanol, 19.2g of 4,4'-bis(3,4,5-trimethoxy)benzoin was obtained, with a yield of 62.8%, m.p: 165.2-165.6°C.
[0047] (2) Preparation of 4,4'-bis(3,4,5-trimethox...
Embodiment 3
[0052] (1) Preparation of 4,4'-bis(3,4,5-trimethoxy)benzoin
[0053] Add vitamin B to the three-neck round bottom flask equipped with reflux condensation, stirring device and thermometer in sequence 1 2g, 5mL of distilled water, 16mL of ethanol, and 15.3g of 3,4,5-trimethoxybenzaldehyde, and slowly stirred in an ice-water bath for 15min. Then add 3.0 mol / L sodium hydroxide ethanol solution dropwise through the condenser to adjust the pH value of the solution to 8, and continuously add 3.0 mol / L sodium hydroxide ethanol solution to keep the pH value of the solution for 45 minutes. After heating to 75°C for 3h, cool to room temperature. After standing for 24 hours, suction filtration, the filter cake was washed 3 times with distilled water, and the filtrate was neutral to obtain a yellow solid. After recrystallization from ethanol, 9.0 g of 4,4'-bis(3,4,5-trimethoxy)benzoin was obtained with a yield of 58.9%, m.p: 165.2-165.6°C.
[0054] (2) Preparation of 4,4'-bis(3,4,5-trim...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com