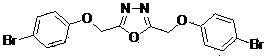
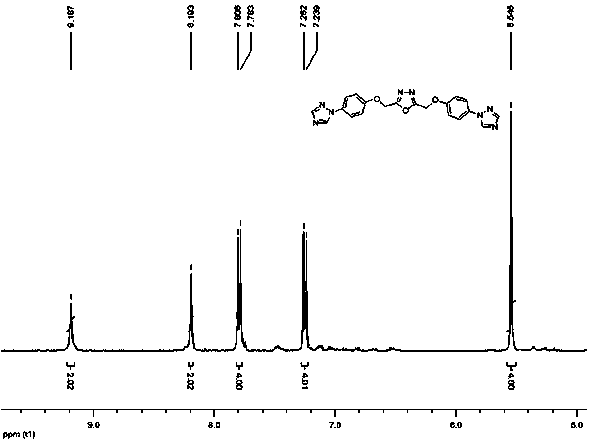
Bitriazole phenyl-substituted oxadiazole compound and preparation method and application thereof
A technology for oxadiazole compounds, which is applied in the field of synthesis of oxadiazole compounds, can solve problems such as undetected and unretrieved, and achieve the effects of high reaction yield, high purity, and high application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] The preparation method of 2,5-bis((4-bromophenoxy)methyl)-1,3,4-oxadiazole:
[0031] In a 50 mL three-neck flask equipped with a magneton and a reflux condenser, add 4-bromophenoxyacetic acid (4.62 g, 20 mmol), 4-bromophenoxyacetylhydrazide (4.90 g, 20 mmol), 20 mL POCl 3, refluxed for 24 hours under the protection of nitrogen, after the reaction was completed, the reaction was lowered to room temperature, poured into ice water, a large amount of precipitate was precipitated, filtered with suction, the filter cake was collected, and recrystallized with water to obtain 2,5-bis((4-bromo Phenoxy)methyl)-1,3,4-oxadiazole, the yield was 89%.
Embodiment 2
[0033] The molar ratio of 2,5-bis((4-bromophenoxy)methyl)-1,3,4-oxadiazole: 1,2,4-triazole: potassium carbonate: cuprous oxide is 2: 10:30:1
[0034] In a 50 mL three-necked round-bottomed flask equipped with a magnet, reflux condenser and thermometer, Cu 2 O (0.0715 mg, 0.5 mmol), potassium carbonate (2.0731 g, 15 mmol), 1,2,4-triazole (0.345 g, 5 mmol), 2,5-bis((4-bromophenoxy) Methyl)-1,3,4-oxadiazole (0.4401 g, 1 mmol), 20 mL DMF. Start stirring at 100°C and react for 24 hours. After the reaction, the reaction solution was lowered to room temperature, filtered, and 100 mL of water was added to the filtrate, a large amount of precipitate was precipitated, filtered with suction, and the filter cake was collected, with a yield of 63%.
[0035] Preparation:
[0036] The molar ratio of 2,5-bis((4-bromophenoxy)methyl)-1,3,4-oxadiazole: 1,2,4-triazole: potassium carbonate: cuprous oxide is 2: 10:30:1
[0037] In a 50 mL three-necked round-bottomed flask equipped with a mag...
Embodiment 3
[0039] The method used as a fungicide is as follows:
[0040] There are two main methods for determining the inhibitory activity of fungicides against fungi: the growth rate method and the spore germination method. The present invention has selected the growth rate method (Sun Jialong, Mu Wei, 2009) i.e. the culture medium dosing method, by mixing the test agent with the unsolidified medium and then connecting the strains, cultivating the pathogenic bacteria with the drug-containing medium, The bactericidal activity of the test agent was judged by the growth rate of the pathogenic bacteria (diameter of the colony). In this paper, the fungicidal activity of target compounds against Botrytis cinerea and Sclerotinia wheat was determined, and carbendazim was used as control.
[0041] compound Virulence regression equation correlation coefficient EC 50 (95% confidence limit) (μg / mL) Compound III Y=3.9747+1.2671X 0.9479 6.44(5.00–8.30) Carbendazim Y...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com