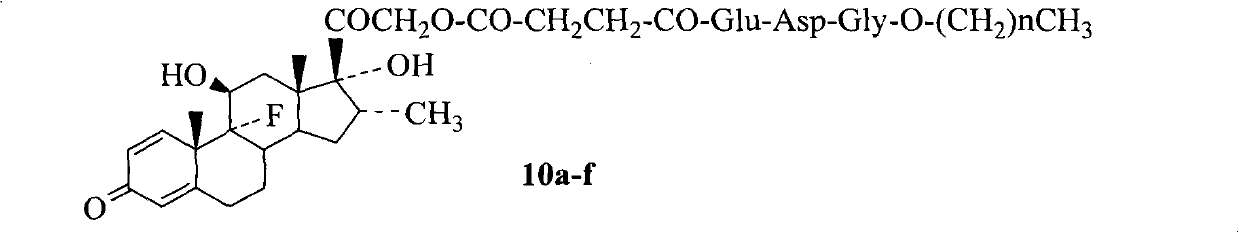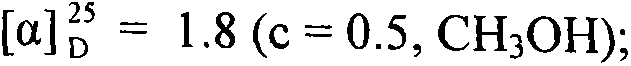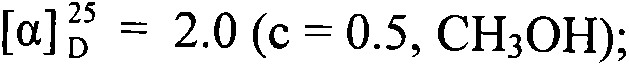Conjugates of saturated aliphatic chain alcohol, dexamethasone, and Glu-Asp-Gly, preparation, nano structure, and applications thereof
A technology of -glu-asp-gly and dexamethasone, applied in the field of structure, can solve the problems of osteoporosis side effects, limited treatment objects and regimens, low bioavailability, etc., and achieves broad application prospects and excellent immunosuppressive effects. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031]Embodiment 1 prepares dexamethasone 3-formyl propionate (1)
[0032] 3.93g (10mmol) dexamethasone reacted with 1.3g (13mmol) succinic anhydride in 150ml tetrahydrofuran (THF) in the presence of 1.46g (12mmol) 4-dimethylaminopyridine (DMAP) in the dark for 48 hours, TLC (CH 2 Cl 2 :CH 3 OH:HOAC, 20:1:0.15) showed complete disappearance of starting material. Add 30ml H to the reaction mixture 2 O, concentrated under reduced pressure, the residue was added KHSO 4 The pH was adjusted to 2, and the precipitated colorless powder was washed with acetone / petroleum ether to obtain 4.64 g (94%) of the title compound as a colorless powder. ES I / MS(m / z)491[M-H] - . 1 H NMR (300MHZ, DMSO-d6): δ / ppm=7.29(d, J=10.2Hz, 1H), 6.23(d, J=9.3Hz, 1H), 6.01(s, 1H), 5.42(s, 1H ), 5.17(s, 1H), 5.05(d, J=17.7Hz, 1H), 4.80(d, J=17.7Hz, 1H), 4.15(m, 1H), 2.88(m, 1H), 2.52(m , 2H), 2.61(m, 3H), 2.34(m, 2H), 2.15(m, 2H), 1.77(m, 1H), 1.61(m, 2H), 1.49(s, 3H), 1.35(m, 1H), 1.08 (m, 1H), 0.88 ...
Embodiment 2
[0033] Embodiment 2 prepares dexamethasone 3-N-formylsuccinimidyl propionate (2)
[0034] 4.92g (10mmol) dexamethasone succinate (1) was present in 2.50g (13mmol) 1-ethyl-(3-dimethylaminopropyl)carbodiimide hydrochloride (HCl·EDC) Under reaction with 1.50g (13mmol) N-hydroxysuccinimide (HOSu) in the solvent of 100ml THF and 10ml DMF dark reaction 24 hours, TLC (CH 2 Cl 2 :CH 3 OH:HOAC, 30:1:0.15) showed complete disappearance of starting material. The reaction mixture was concentrated under reduced pressure, 150ml of ethyl acetate was added to the residue, and the resulting solution was washed with 20ml of saturated NaHCO 3 Wash 3 times with aqueous solution, wash 2 times with 20ml saturated NaCl aqueous solution, wash with 20ml saturated KHSO 4 The aqueous solution was washed 3 times, then washed 2 times with 20ml saturated NaCl aqueous solution, the ethyl acetate layer was dried with anhydrous sodium sulfate, filtered, the filtrate was concentrated to dryness under reduc...
Embodiment 3
[0035] Embodiment 3 prepares Boc-Gly-O-(CH 2 ) 7 CH 3 (3a)
[0036] 0.875 g (5 mmol) of Boc-Gly was dissolved in 20 mL of anhydrous THF, and 0.675 g (5 mmol) of N-hydroxybenzotriazole (HOBt) was added to the resulting solution under ice cooling to completely dissolve it. After 10 minutes, 1.236 g (6 mmol) of dicyclohexylcarbodiimide (DCC) was added to obtain reaction solution I. Put 0.78g (6mmol) CH in ice bath 3 (CH 2 ) 7 OH was suspended in 20 mL of anhydrous dichloromethane, and then 1 mL of N-methylmorpholine (NMM) was added to adjust the pH to 9. Stir for 35 minutes to obtain reaction solution II. The reaction solution I was added to the reaction solution II under ice-cooling, first stirred under ice-cooling for 1 h, and then stirred at room temperature for 12 h, TLC (ethyl acetate:petroleum ether, 2:1) showed that Boc-Gly disappeared. Dicyclohexylurea (DCU) was filtered off, concentrated under reduced pressure, and the residue was dissolved in 50 mL of ethyl acet...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com