Preparation method for lithium-enriched manganese-based cathode material
A cathode material, lithium-rich manganese-based technology, applied in battery electrodes, electrical components, circuits, etc., can solve problems such as loss of metal ions, large amounts of waste water, etc., and achieve good electrochemical performance.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
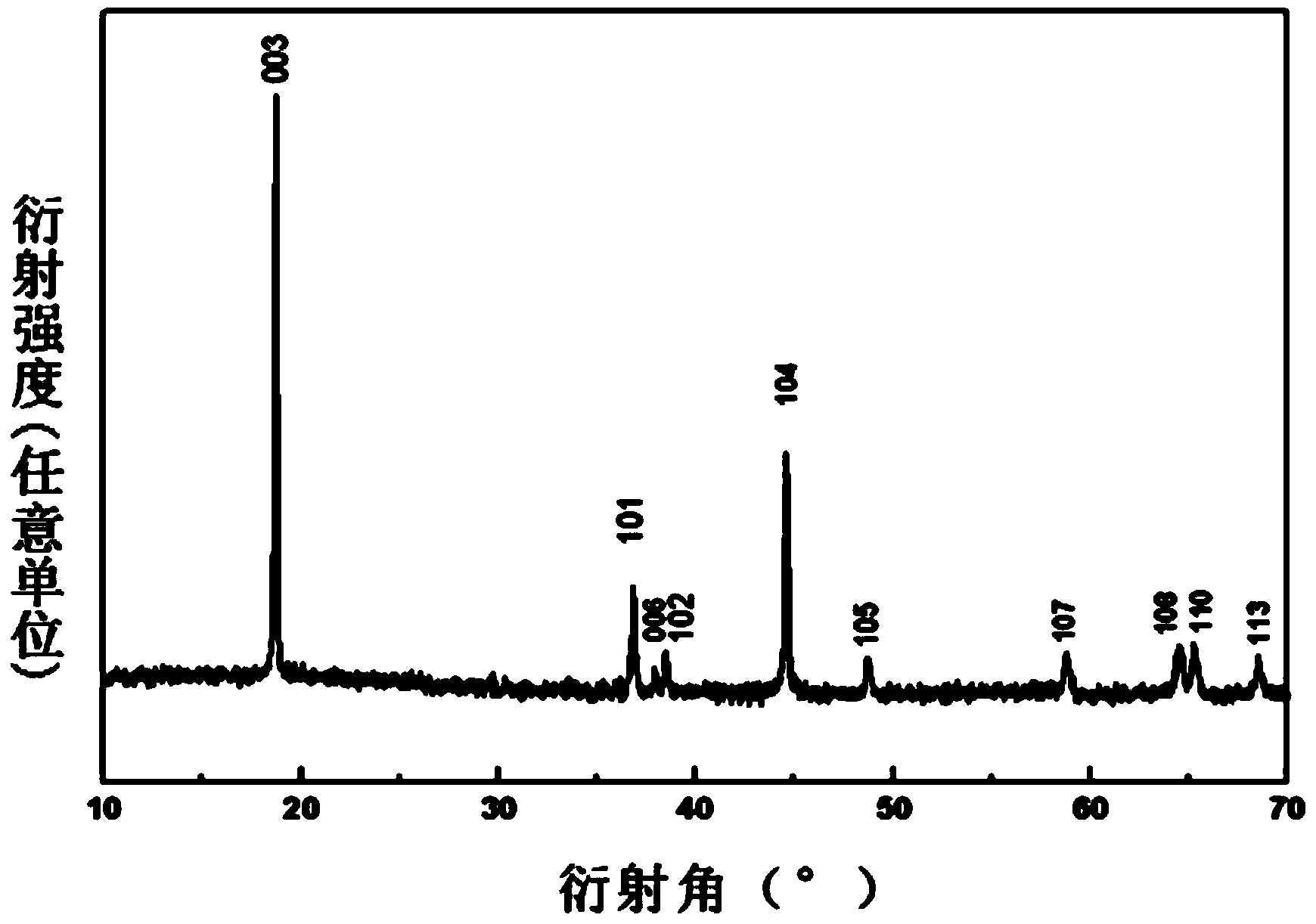
Image
Examples
preparation example Construction
[0035] In the first aspect, an embodiment of the present invention provides a method for preparing a lithium-rich manganese-based positive electrode material, including:
[0036] Step 101, according to the chemical formula Li 1.5 Ni 0.25-x mn 0.75-x m 2x o 2.5 The stoichiometric ratio, prepare the aqueous solution of the mixture of lithium salt, nickel salt, manganese salt and M salt, and add a predetermined amount of urea to the aqueous solution of the mixture of lithium salt, nickel salt, manganese salt and M salt, mix well , to obtain a mixed solution, wherein, the chemical formula Li 1.5 Ni 0.25-x mn 0.75-x m 2x o 2.5 Among them, 0.003≤x≤0.02, M is cobalt or chromium.
[0037] Step 102, reacting the mixed solution in a sealed container at 70-90° C. under stirring condition to obtain a reaction system containing the first precipitate.
[0038] Step 103, under stirring conditions, add (NH 4 ) 2 CO 3 After the reaction is complete, a reaction system containing th...
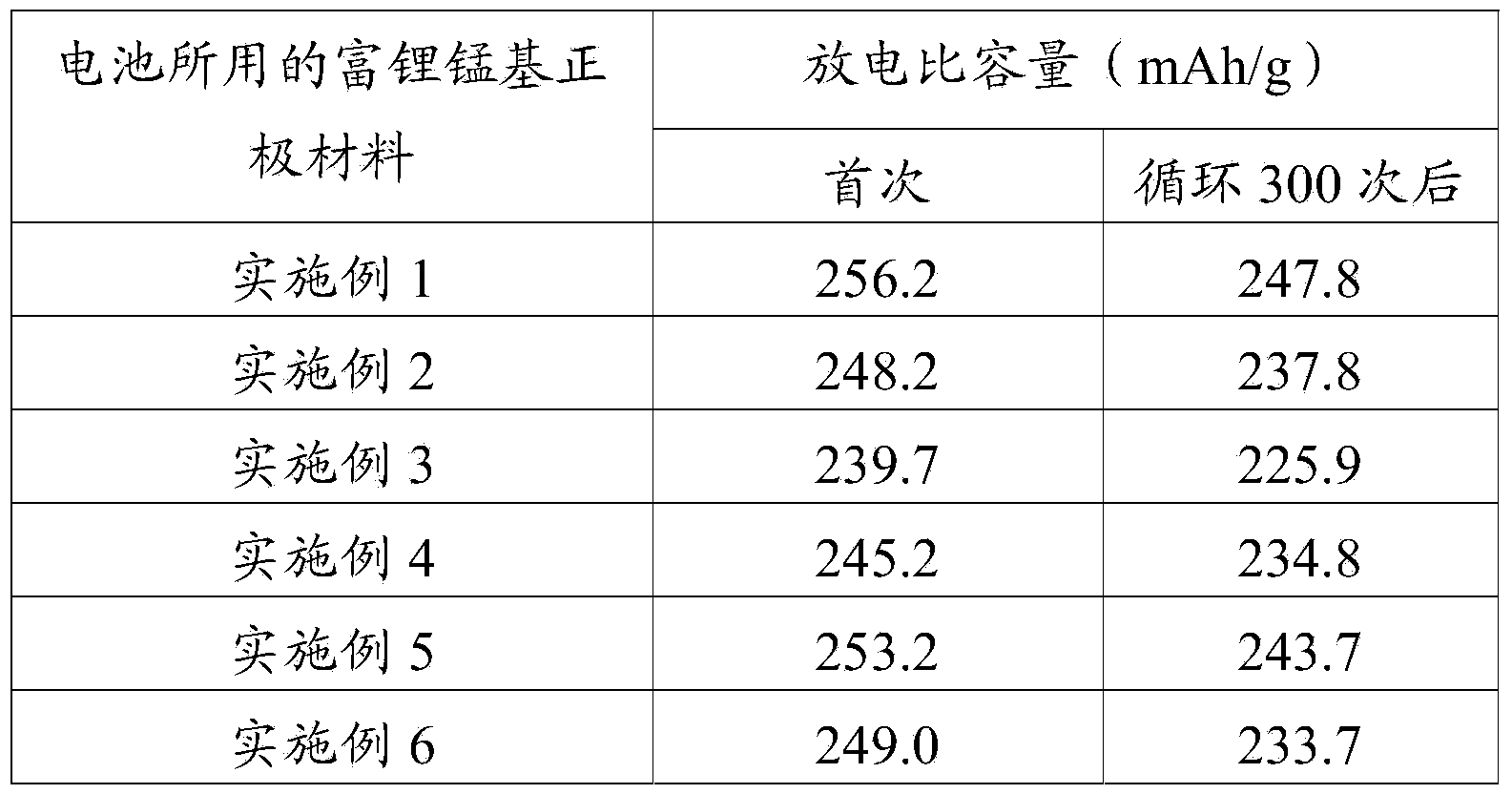
Embodiment 1
[0065] According to the chemical formula Li 1.5 Ni 0.244 mn 0. 7 44 Cr 0.012 o 2.5 The stoichiometric ratio, the aqueous solution of the mixture of preparation lithium nitrate, nickel nitrate, manganese nitrate and chromium nitrate, press (nickel ion+manganese ion+chromium ion): urea=1:3 material ratio, add in this aqueous solution Urea, after ultrasonic stirring for 3 hours (the sound intensity of ultrasonic wave is 60W / cm 2 ) were mixed uniformly to obtain a mixed solution. at 150r p At a stirring speed of m, the mixed solution was reacted in a sealed container at 70° C. for 10 h, until no precipitation occurred, and cooled naturally to obtain a reaction system containing the first precipitate. According to (NH 4 ) 2 CO 3 The molar ratio of the carbonate group in the aqueous solution to the lithium ion in the lithium salt is 1.1:2. Under the condition of ultrasonic stirring, the reaction system containing the first precipitate is added dropwise (NH 4 ) 2 CO 3 Wh...
Embodiment 2
[0068] According to the chemical formula Li 1.5 Ni 0.247 mn 0.747 co 0.006 o 2.5 The stoichiometric ratio, the aqueous solution of the mixture of preparation lithium acetate, nickel acetate, manganese acetate and cobalt acetate, according to (nickel ion+manganese ion+cobalt ion): urea=1:2 substance molar ratio, after ultrasonic stirring 2 hours (Ultrasonic sound intensity is 75W / cm 2 ) were mixed uniformly to obtain a mixed solution. At a stirring speed of 100 rpm, the mixed solution was reacted in a sealed container at 80° C. for 8 hours until no precipitation occurred, and cooled naturally to obtain a reaction system containing the first precipitate. According to (NH 4 ) 2 CO 3 The molar ratio of the carbonate group in the aqueous solution to the lithium ion in the lithium salt is 1.1:2. Under the condition of ultrasonic stirring, the reaction system containing the first precipitate is added dropwise (NH 4 ) 2 CO 3 When the aqueous solution is reacted to the surfa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com