Preparation method for 3-aminomethyl-3,5,5-trimethylcyclohexyl amine
A technology of trimethylcyclohexylamine and trimethylcyclohexanone, which is applied in the field of preparation of aliphatic amines, can solve the problems of reduced conversion rate, device shutdown, and increased residence time of hydrogenation catalyst loading, so as to avoid Decomposition, the effect of ensuring stable operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
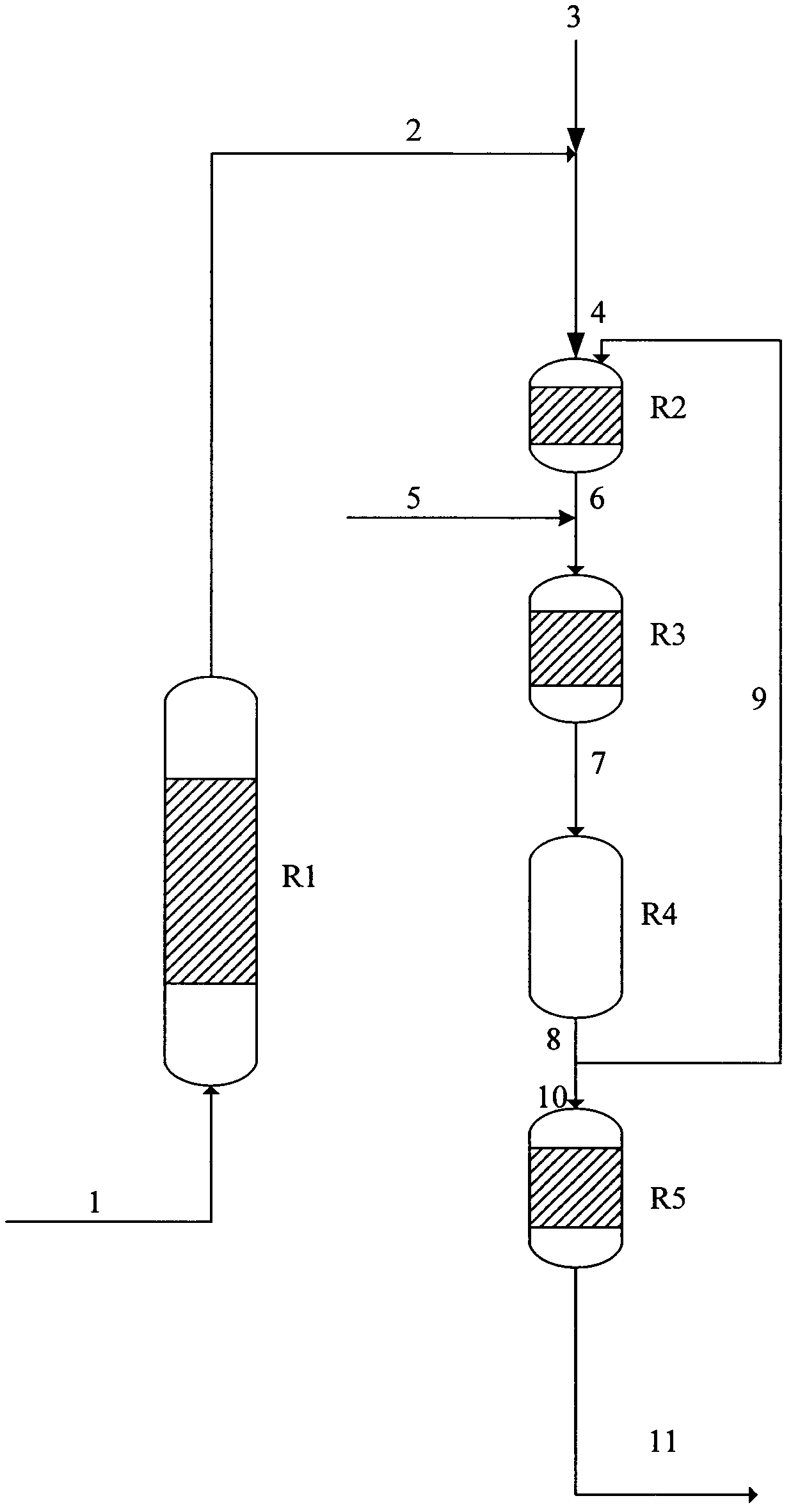
[0062] process such as figure 1 As shown, R1 is an imidization reactor, which uses a tubular reactor, and the catalyst inside the reactor is 75mm high and 20mm in diameter; R2 is the first-stage hydrogenation reactor, which uses a trickle bed reactor, and the catalyst is filled inside the reactor 75mm in height and 20mm in diameter; R3 is the second-stage hydrogenation reactor, which adopts a trickle bed reactor, and the catalyst inside the reactor is 75mm in height and 20mm in diameter; R4 is the auxiliary agent decomposition reactor, and the catalyst in the reactor is 400mm in height. The diameter is 20mm, and R5 is the third-stage hydrogenation reactor, which adopts a trickle bed reactor. The catalyst inside the reactor is 37.5mm high and 20mm in diameter.
[0063] R1 is filled with γ-Al with a diameter of 0.5mm 2 o 3 Balls, R2, R3, R5 are each equipped with 16-30 mesh Raney cobalt catalyst Catalloy 6400 for block fixed bed, R4 is filled with about 0.5mm α-Al 2 o 3 roun...
Embodiment 2
[0067] Other conditions are as embodiment 1, change the hydrogenation reaction auxiliary agent wherein into 5wt% tetraethylammonium hydroxide methanol solution, feed rate is 16g / h, R3 reaction temperature is changed into 100 ℃, R4 decomposition temperature is changed into At 135°C, the circulating stream 9 accounts for 4wt% of the weight of the aid decomposition reaction material 8.
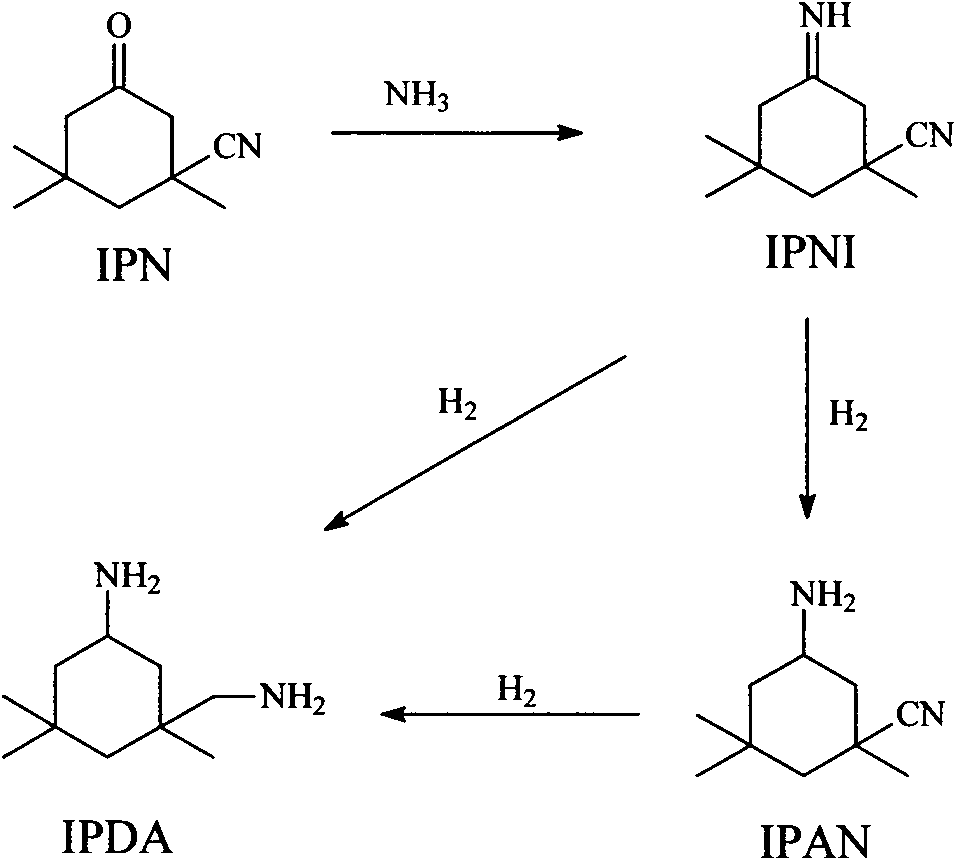
[0068] Samples were taken from the outlet of the R1 reactor, and the content of IPNI in the imidization reaction material 2 was about 95 wt%, and samples were taken from the outlet of the R2 reactor, and the content of IPN in the first hydrogenation reaction material 6 was about 0.005 wt%. Sampling from the outlet of the R3 reactor, the content of IPDA in the second hydrogenation reaction material 7 is about 85wt%, and the content of IPAN is about 13.5wt%; sampling analysis from the outlet of the R4 reactor does not detect tetraethylammonium hydroxide, and reacts from R5 Sampling analysis at the ...
Embodiment 3
[0070] Other conditions are as embodiment 1, change the hydrogenation reaction auxiliary agent wherein into 1wt% tetramethylammonium hydroxide aqueous solution, feed rate is 16g / h, R4 decomposition temperature is changed into 140 DEG C, and circulation stream 9 accounts for auxiliary The agent decomposes 3wt% of reaction material 8 weights.
[0071] Samples were taken from the outlet of the R1 reactor, and the content of IPNI in the imidization reaction material 2 was about 95 wt%, and samples were taken from the outlet of the R2 reactor, and the content of IPN in the first hydrogenation reaction material 6 was about 0.004 wt%. Sampling from the outlet of the R3 reactor, the content of IPDA in the second hydrogenation reaction material 7 is about 86.5wt%, and the content of IPAN is about 12wt%; sampling and analysis from the outlet of the R4 reactor, no tetramethylammonium hydroxide is detected, and the reaction from R5 Sampling analysis at the outlet of the reactor shows that...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com