bafeo doped with ca element at b site 3-δ Ceramic based oxygen permeable membrane material
A bafeo3-, bafe1-xcaxo3- technology, applied in the field of inorganic oxygen permeable membrane materials, can solve the problems of high valence, reduce the concentration of oxygen vacancies in the material, increase the production cost of materials, etc., and achieve the goal of improving oxygen permeability and reducing costs Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
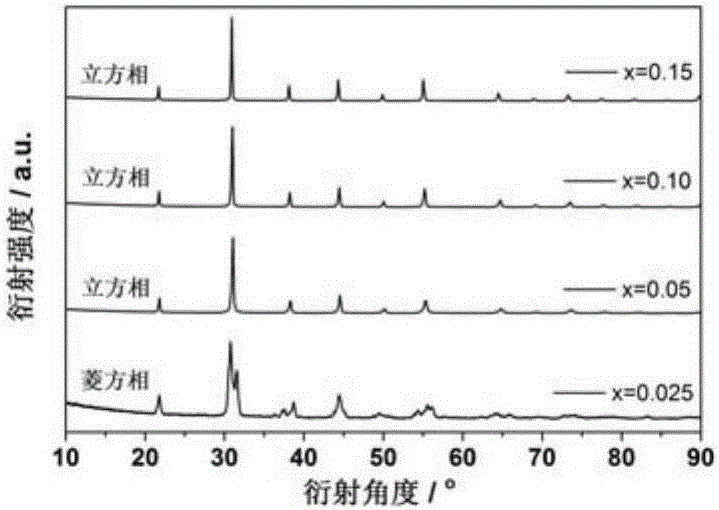
[0015] Example 1: BaFe 0.975 Ca 0.025 o 3- δ Citric acid-nitrate synthesis
[0016] a) 10.454g BaNO 3 , 15.756gFe(NO 3 ) 3 9H 2 O, 0.236gCa(NO 3 ) 2 4H 2 O was dissolved in 500 mL of ionized water, and 50 mL of 65 wt% HNO was added 3 , and stirred for 2 hours.
[0017] b) Add 23.379g of ethylenediaminetetraacetic acid and 25.217g of citric acid to the above solution, adjust the pH value of the solution to 5 with 28wt% ammonia water, and stir for 12 hours.
[0018] c) Subsequently, the mixed solution was evaporated in a water bath at 60° C. to obtain a colloid, and the colloid was moved to an oven and ignited at 200° C. to obtain a precursor powder.
[0019] d) Treat the precursor in an air atmosphere at 800°C for 8 hours. After cooling to room temperature, transfer it to a mortar, add 1wt.% PVA and mix evenly. Use a mold to dry press the mixed precursor under a pressure of 100MPa . The dense samples were obtained by sintering at 1300℃ for 6h in air atmosphere. S...
Embodiment 2
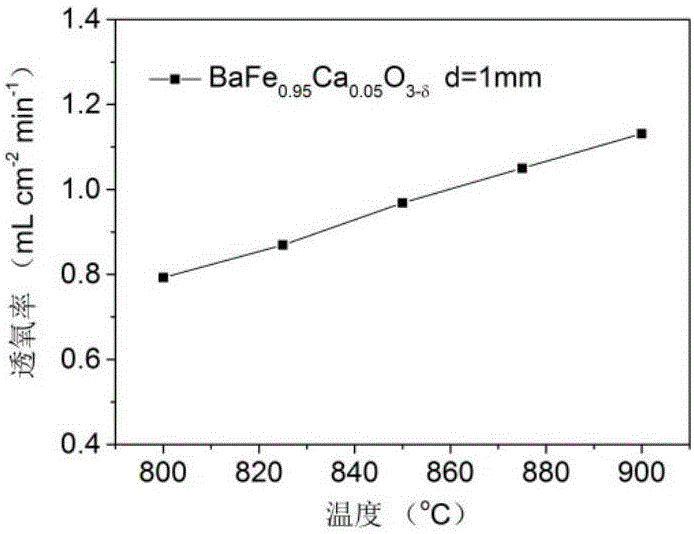
[0020] Example 2: BaFe 0.95 Ca 0.05 o 3- δ Citric acid-nitrate synthesis
[0021] a) 10.454g BaNO 3 , 15.352gFe(NO 3 ) 3 9H 2 O, 0.472gCa(NO 3 ) 2 4H 2 O was dissolved in 500 mL of ionized water, and 50 mL of 65 wt% HNO was added 3 , and stirred for 2 hours.
[0022] b) Add 23.379g of ethylenediaminetetraacetic acid and 25.217g of citric acid to the above solution, adjust the pH value of the solution to 7 with 28wt% ammonia water, and stir for 12 hours.
[0023] c) Subsequently, the mixed solution was evaporated in a water bath at 100° C. to obtain a colloid, and the colloid was moved to an oven and ignited at 300° C. to obtain a precursor powder.
[0024] d) Treat the precursor in an air atmosphere at 700°C for 8 hours. After cooling to room temperature, transfer it to a mortar, add 3wt.% PVA and mix evenly. Use a mold to dry press the mixed precursor under a pressure of 150MPa . Sintering at 1350°C for 6 hours in an air atmosphere gave a dense sample without in...
Embodiment 3
[0025] Example 3: BaFe 0.90 Ca 0.10 o 3- δ Citric acid-nitrate synthesis
[0026] a) 10.454g BaNO 3 , 14.544gFe(NO 3 ) 3 9H 2 O, 0.944gCa(NO 3 ) 2 4H 2 O was dissolved in 500 mL of ionized water, and 50 mL of 65 wt% HNO was added 3 , and stirred for 2 hours.
[0027] b) Add 23.379g of ethylenediaminetetraacetic acid and 25.217g of citric acid to the above solution, adjust the pH value of the solution to 10 with 28wt% ammonia water, and stir for 12 hours.
[0028] c) Subsequently, the mixed solution was evaporated in a water bath at 90° C. to obtain a colloid, and the colloid was moved to an oven and ignited at 200° C. to obtain a precursor powder.
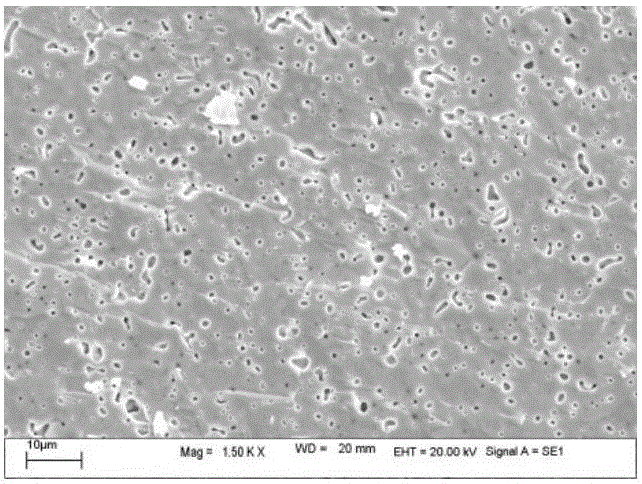
[0029] d) Treat the precursor in an air atmosphere at 900°C for 8 hours. After cooling to room temperature, transfer it to a mortar, add 5wt.% PVA and mix evenly. Use a mold to dry press the mixed precursor under a pressure of 200MPa . Such as image 3 As shown, sintering at 1150°C for 6 hours in an air atmosphere yie...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com