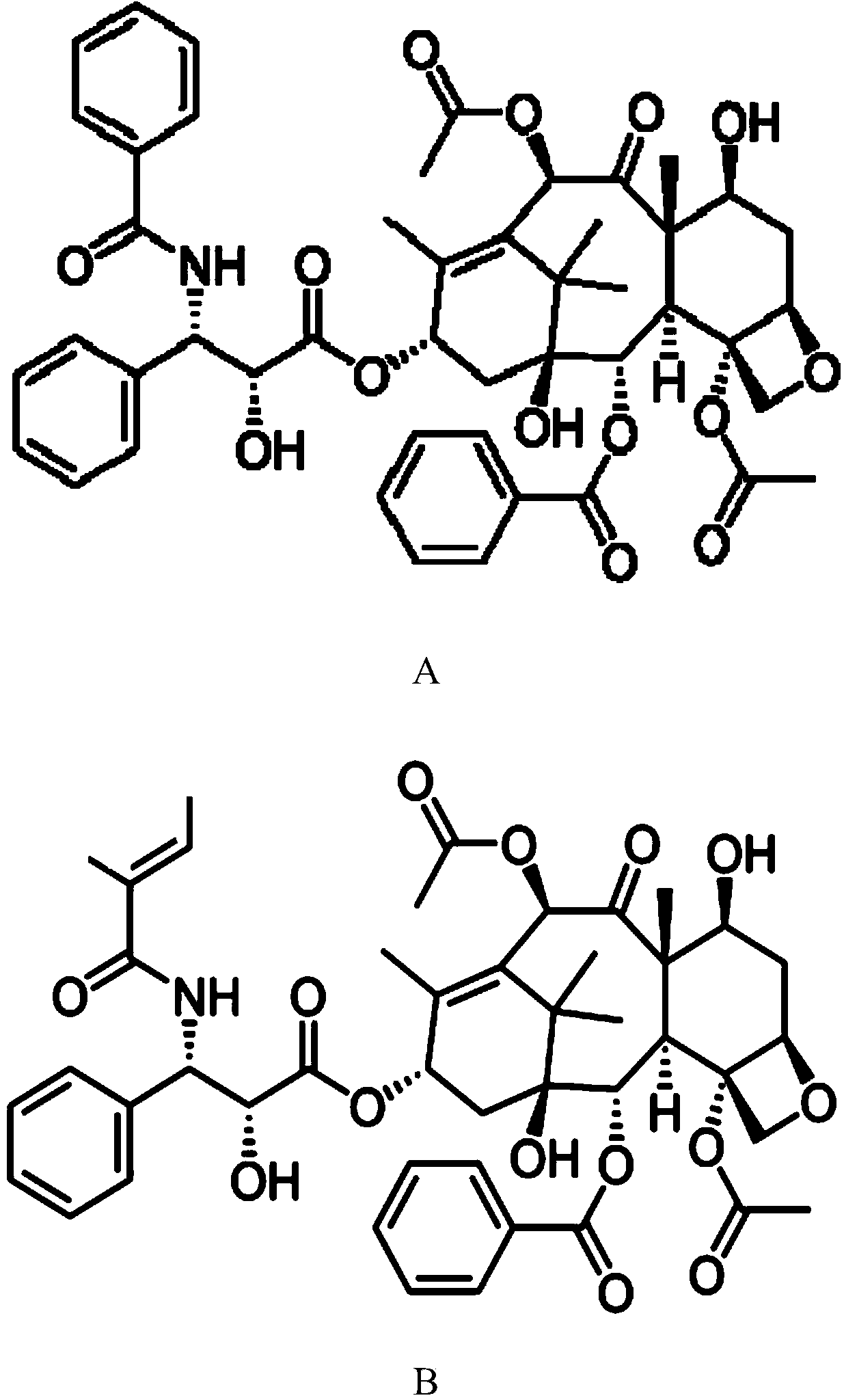
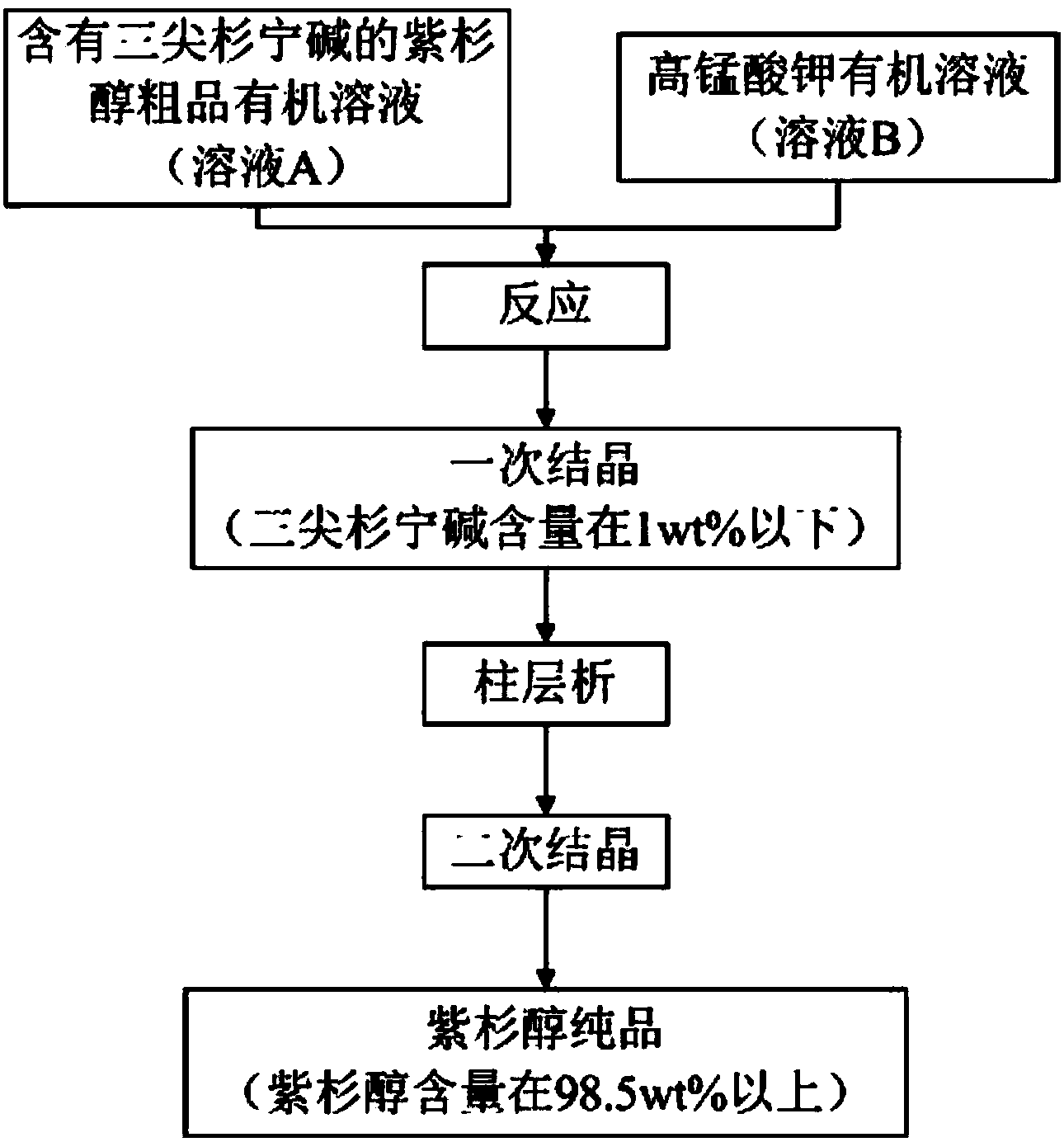
Method for removing cephalomannine from paclitaxel
A technology of cephalomannine and paclitaxel, which is applied in the field of separation and purification of paclitaxel, can solve the problems of expensive ozone generator equipment, relatively harsh conditions, and high production costs, and achieve mild reaction and treatment conditions, good removal effect, and high purity and the effect of high recovery rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] (1) Take 50 g of paclitaxel crude product (paclitaxel content 47.6wt%, cephalomannine content 10.1wt%) with 300ml acetone and add potassium permanganate solution (2.5g add 100ml acetone to dissolve) to react. After reacting for 3 hours, filter to obtain about 450ml of filtrate, add 250ml of purified water to crystallize, and dry the crystals to obtain 31.3g. According to HPLC detection, the content of paclitaxel is 74.2%, the content of cephalomannine is 0.44%, and the yield of paclitaxel is 97.6%.
[0041] (2) Dissolve the product from the previous step with dichloromethane to obtain a wet sample, add 360g of silica gel to a 50×500 glass column, and moisten the column with dichloromethane, then add the wet sample to the head of the column, and use the volume of ethyl acetate The mixed solvent of ethyl acetate and dichloromethane with a percentage of 30% was used as the mobile phase for elution, and the main segment was collected, a total of 1.5L. After concentration and...
Embodiment 2
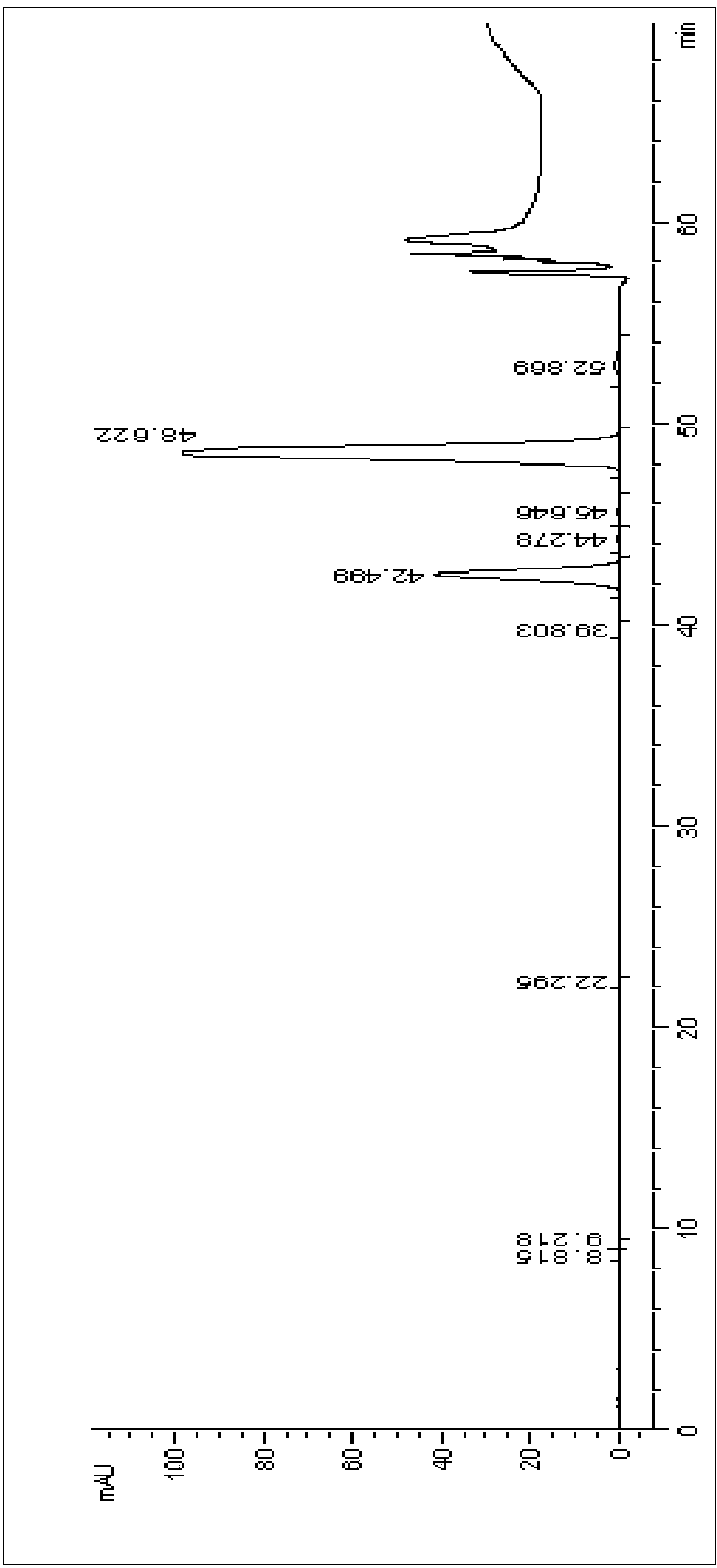
[0043] (1) get paclitaxel crude product 300g (paclitaxel content 52.8wt%, cephalomannine content 16.1wt%, the liquid chromatogram of its efficient separation preparation is as follows image 3 Shown, the peak at 48.6 place is the characteristic peak of paclitaxel, the peak at 42.4 place is the characteristic peak of cephalomannine) add potassium permanganate solution (7g adds 900ml acetone to dissolve) after dissolving with 3000ml acetone and react. After reacting for 8 hours, filter to obtain about 4000ml of filtrate, add 7000ml of purified water to crystallize, and dry the crystals to obtain 203.2g. HPLC detection, the detection results are as follows: Figure 4 Shown, the peak at 48.1 place is the characteristic peak of paclitaxel, and the peak at 42.1 place is the characteristic peak of cephalomannine, and after analysis, obtaining the paclitaxel content is 76.7%, and the cephalomannine content is 0.58%, and the paclitaxel yield is 98.4%.
[0044] (2) Dissolve the product...
Embodiment 3
[0046] (1) 1000 g of paclitaxel crude product (paclitaxel content 50.1 wt%, cephalomannine content 19.8 wt %) was dissolved in 8 L of acetone, and potassium permanganate solution was added (60 g was dissolved in 2 L of acetone) for reaction. After reacting for 5 hours, filter to obtain about 10.5 L of filtrate, add 10 L of purified water to crystallize, and dry the crystals to obtain 690.3 g. According to HPLC detection, the content of paclitaxel is 71.2%, the content of cephalomannine is 0.67%, and the yield of paclitaxel is 98.1%.
[0047] (2) Dissolve the product from the previous step with dichloromethane to obtain a wet sample, add 45kg of silica gel to the 400×1000 stainless steel column, moisten the column with dichloromethane, then add the wet sample to the head of the column, and use the volume of ethyl acetate The mixed solvent of ethyl acetate and dichloromethane with a percentage of 20% was used as the mobile phase for elution, and the main segment was collected, wi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com