Relay-type multifunctional fluorescent probe, and preparation method and application thereof
A fluorescent probe, multifunctional technology, applied in fluorescence/phosphorescence, chemical instruments and methods, luminescent materials, etc., to achieve good sensitivity, excellent water solubility, and easy separation and purification of products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Example 1: Preparation of a relay-type multifunctional fluorescent probe
[0030] (1) The reaction formula of the synthetic relay type multifunctional fluorescent probe:
[0031]
[0032] (2) Concrete steps for synthesizing relay-type multifunctional fluorescent probes:
[0033] Weigh 0.400g of N,N'-((1,3,4-oxadiazol-2,5-yl)bis(2,1-phenyl))bis(2-chloroacetamide), 0.391g di -(Pyridylmethylene)amine, 1.5mL N,N-diisopropylethylamine and 0.104g potassium iodide, dissolved in 140mL dry acetonitrile, stirred and heated to reflux for 10h under the protection of nitrogen, wherein the heating and reflux temperature was 82°C The solvent is evaporated under reduced pressure at the end of the reaction, and the residue is separated by column chromatography with the eluent of ethyl acetate and methanol with a volume ratio of 10: 1 to obtain the relay type multifunctional fluorescent probe N, N'-(( 1,3,4-oxadiazol-2,5-yl)bis(2,1-phenyl))bis(2-(bis(pyridine-2-methylene)amino)aceta...
Embodiment 2
[0034] Example 2: Preparation of a relay-type multifunctional fluorescent probe
[0035] Weigh 0.400g of N,N'-((1,3,4-oxadiazol-2,5-yl)bis(2,1-phenyl))bis(2-chloroacetamide), 0.391g di -(pyridylmethylene)amine, 1.8mL of N,N-diisopropylethylamine and 0.104g of potassium iodide, dissolved in 140mL of dry acetonitrile, stirred and heated to reflux for 6h under the protection of nitrogen, wherein the temperature of heating to reflux was 82°C The solvent is evaporated under reduced pressure at the end of the reaction, and the residue is separated by column chromatography with the eluent of ethyl acetate and methanol with a volume ratio of 10: 1 to obtain the relay type multifunctional fluorescent probe N, N'-(( 1,3,4-oxadiazol-2,5-yl)bis(2,1-phenyl))bis(2-(bis(pyridine-2-methylene)amino)acetamide), the yield is 65%.
Embodiment 3
[0036] Example 3: Preparation of a relay-type multifunctional fluorescent probe
[0037] Weigh 0.400g of N,N'-((1,3,4-oxadiazol-2,5-yl)bis(2,1-phenyl))bis(2-chloroacetamide), 0.391g di -(Pyridylmethylene)amine, 1.8mL N,N-diisopropylethylamine and 0.104g potassium iodide, dissolved in 140mL dry acetonitrile, stirred and heated to reflux for 10h under the protection of nitrogen, wherein the heating and reflux temperature was 82°C The solvent is evaporated under reduced pressure at the end of the reaction, and the residue is separated by column chromatography with the eluent of ethyl acetate and methanol with a volume ratio of 10: 1 to obtain the relay type multifunctional fluorescent probe N, N'-(( 1,3,4-oxadiazol-2,5-yl)bis(2,1-phenyl))bis(2-(bis(pyridine-2-methylene)amino)acetamide), the yield is 72%.
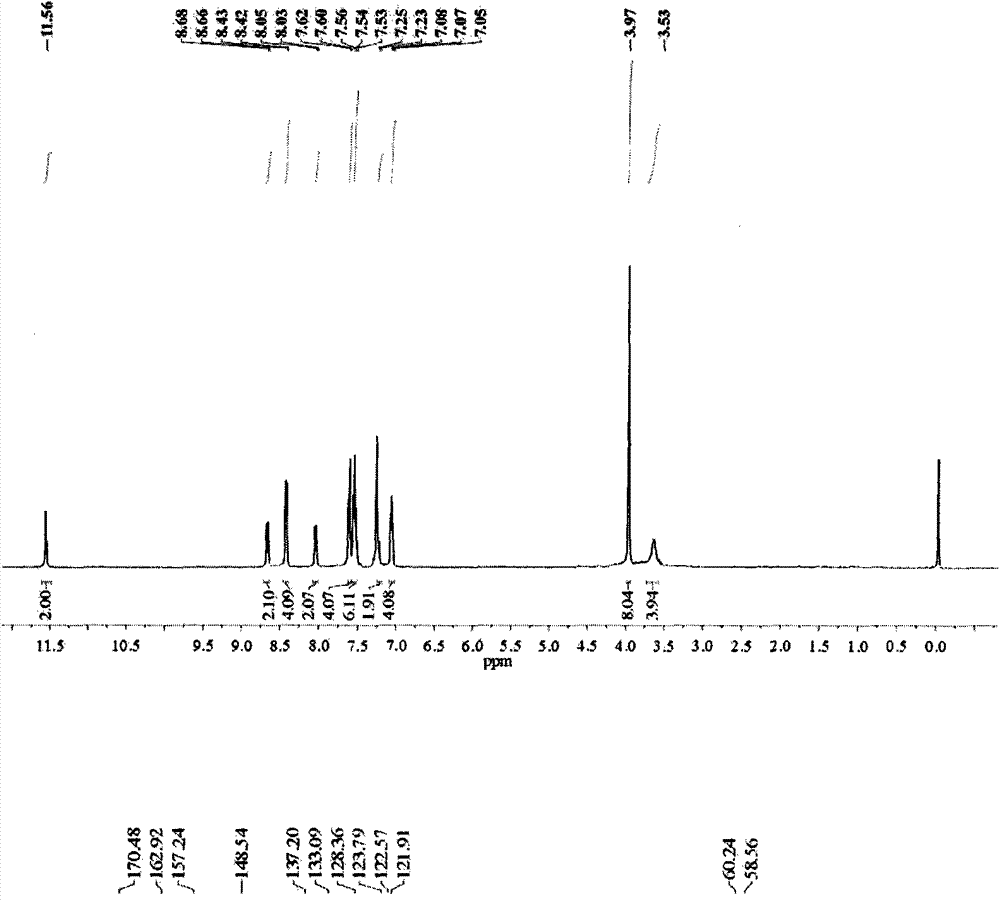
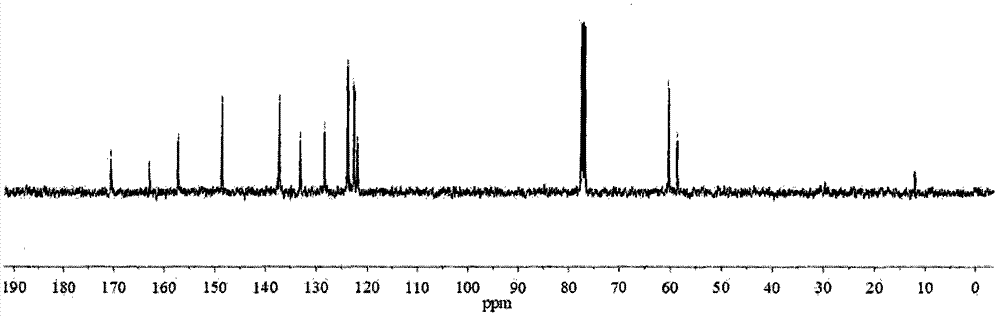
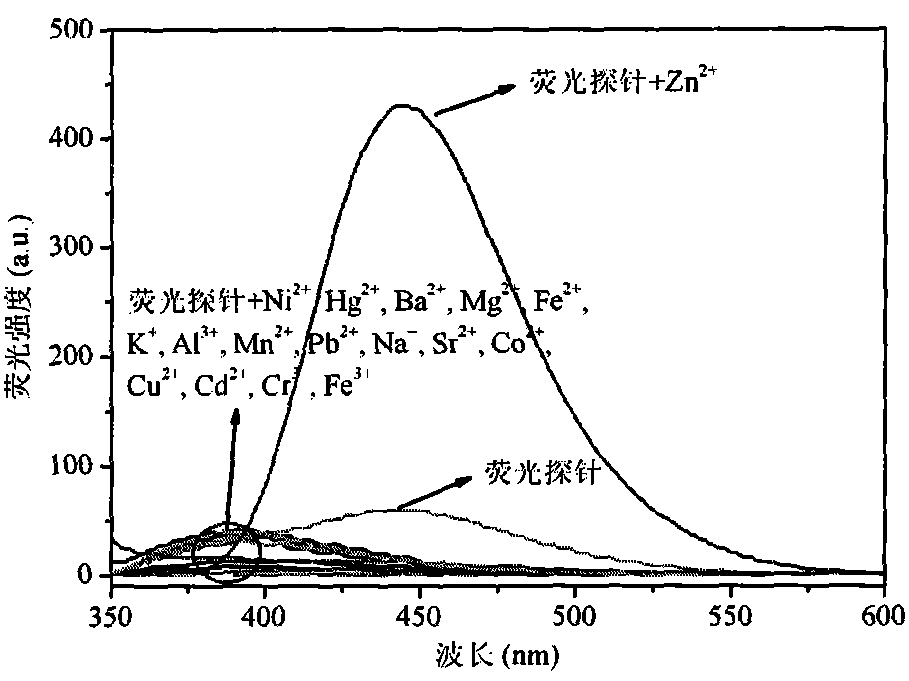
[0038] The basic data of the relay-type multifunctional fluorescent probe N-(2-(1H-benzimidazolyl)phenyl)-2-bis(2-pyridylmethylamino)acetamide prepared in Examples 1 to 3 are...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com