Application of cycloaltin-type triterpene compounds in the preparation of anti-lung cancer drugs
A cycloaltin-type, anti-lung cancer drug technology, applied in the direction of active ingredients of hydroxyl compounds, drug combinations, anti-tumor drugs, etc., to achieve the effect of inhibiting proliferation and rich in plant sources
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
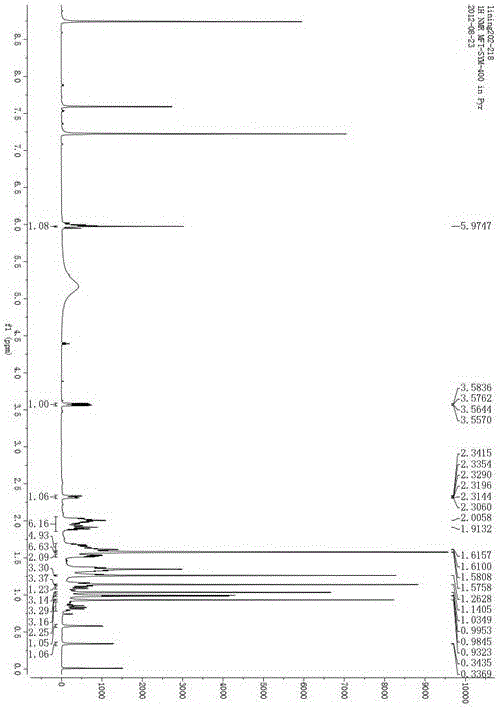
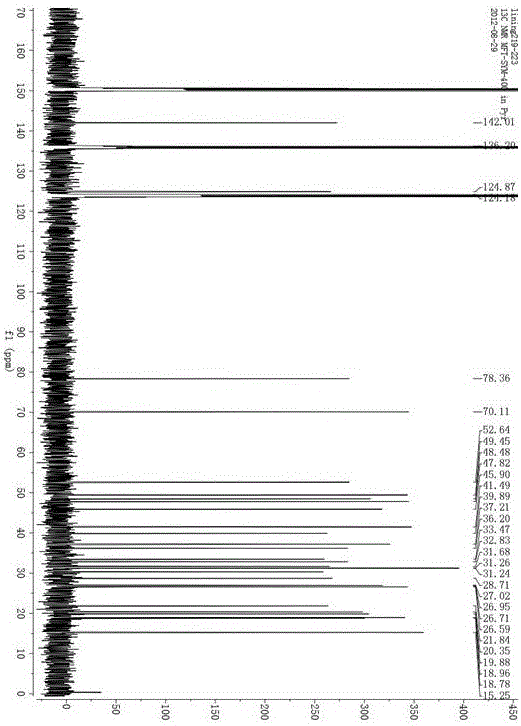
Image
Examples
Embodiment 1
[0012] a. Take the dried and pulverized aerial part of the whole leaf of Indigo spinosa, soak it in 6-10 times the amount of ethanol or methanol with a volume fraction of 50-100%, and then heat and reflux for extraction. The extraction time is 2-3 hours each time, and the extraction times are 2-2 3 times, combined extracts, decompressed recovery extracts to alcohol concentration lower than 5%;
[0013] b. extract the resulting extract through petroleum ether, n-hexane or cyclohexane for 2 to 5 times, the volume ratio of the extraction solvent to the extract is 1 to 3: 3 to 1, combine the extracts, and recover the solvent to obtain the extract;
[0014] c. The obtained extract was separated by silica gel column chromatography, and the mixed solvent of petroleum ether and ethyl acetate was used for gradient elution, and the eluted fraction with a volume ratio of petroleum ether and ethyl acetate of 100:6~8 was collected;
[0015] d. The solvent was distilled off under reduced pr...
Embodiment 2
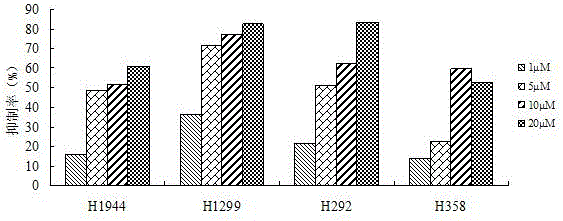
[0028]The cycloaltin-type triterpenoid compound (23Z)-9,19-cycloaltin-23-ene-3α,25-diol isolated in Example 1 was determined by the above-mentioned MTT method for the effect of the compound on the lung cancer cell line H1944 Inhibition experiment steps were carried out, and the results showed that its inhibitory effect on lung cancer cell line H1944 IC 50 The value is 7.8 μmol / L.
Embodiment 3
[0030] The cycloaltin-type triterpenoid compound (23Z)-9,19-cycloaltin-23-ene-3α,25-diol isolated in Example 1 was determined by the above-mentioned MTT method for the effect of the compound on lung cancer cell line H292 Inhibition experiment steps were carried out to measure its inhibitory effect on the proliferation of lung cancer cell line H292 IC 50 The value is 4.9 μmol / L.
[0031] According to the above-mentioned MTT method, the compound is tested against the lung cancer cell line inhibition test steps, and its IC for inhibiting the proliferation of the lung cancer cell line H358 is measured. 50 The value is 8.7 μmol / L.
[0032] The inhibitory effects of the compound (23Z)-9,19-cycloaltin-23-ene-3α,25-diol obtained in the examples of the present invention on four lung cancer cell lines are as follows: image 3 shown.
[0033] The compound is (23Z)-9,19-cycloaltin-23-ene-3α,25-diol, which can be mixed with pharmaceutically acceptable drug excipients to form various for...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com