Direct synthesis of cellulose esters soluble in acetone and products thereof
A technology of cellulose ester and synthesis method, applied in the direction of artificial filament made of cellulose derivatives, etc., can solve problems such as limiting large-scale application, and achieve the effects of saving reaction time, reducing degradation reaction, and improving thermal stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
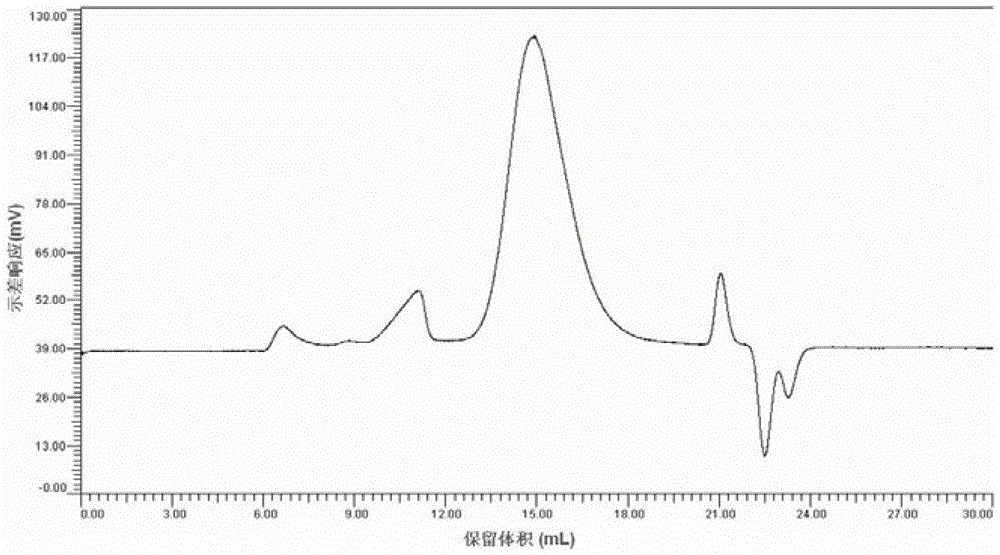
[0065] Put 3g (0.0185mol) cellulose acetate grade hardwood pulp and 150g trifluoroacetic acid into a three-neck flask with mechanical stirring, stir and infiltrate in an ice bath for 2-3 hours, then add 8.6g (0.0843mol) acetic anhydride until transparent state, kept at 50°C for 2 hours, finished the reaction by adding 30g of acetic acid, transferred to a rotary evaporator at 50°C for 1 hour, and finally precipitated the resulting product in a mixed solvent of acetic acid / water, washed with water, and dried to obtain 3.9g of white powder . The substituted acetic acid value (AV) of this product determined by titration method is 55.45%. through 1 The degree of substitution (DS) was determined to be 2.43 by H-NMR. The product is soluble in 91% / 9% dichloromethane / methanol, acetone, dimethylsulfoxide and pyridine.
[0066] The GPC figure of the product prepared by the method described in embodiment 1 and the cellulose diacetate prepared by usual industrial production is attached ...
Embodiment 2
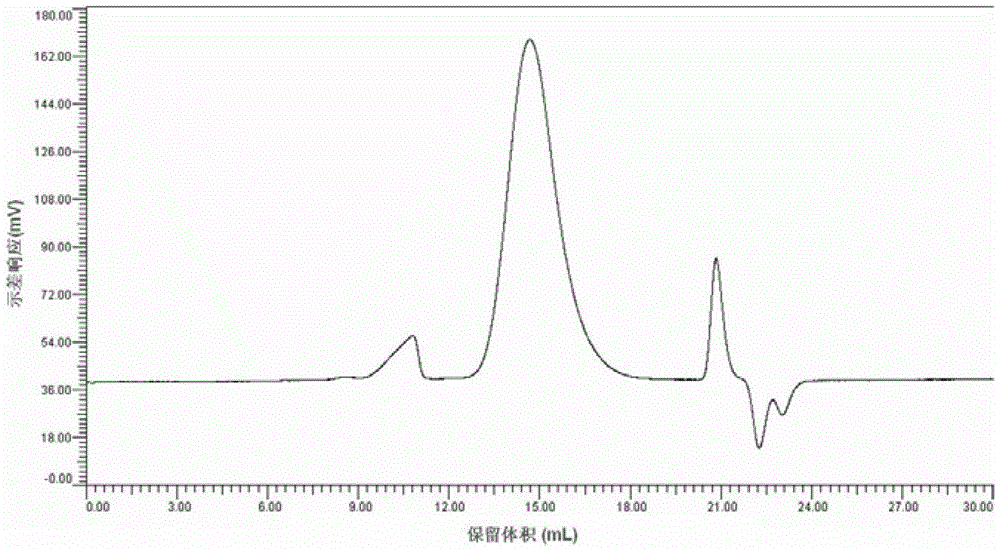
[0072] Put 3 g of cellulose acetate grade hardwood pulp and 150 g of trifluoroacetic acid into a three-neck flask with mechanical stirring, stir and infiltrate in an ice bath for 2-3 hours, then add 8.94 g (0.0877 mol) of acetic anhydride to a transparent state, and keep for 50 After reacting at ℃ for 2 hours, 30 g of acetic acid was added to complete the reaction, and then transferred to a rotary evaporator at 50 °C for 1 hour. Finally, the resulting product was precipitated in an acetic acid / water mixed solvent, washed with water, and dried to obtain 4.3 g of a white powder product. After analysis, the acetic acid value (AV) of this product is 56.54%. 1 The degree of substitution (DS) was determined to be 2.51 by H-NMR. The product has been tested by GPC and FT-IR to prove that it does not contain sulfonate and trifluoroacetate groups. The product is soluble in 91% / 9% dichloromethane / methanol, acetone, dimethylsulfoxide and pyridine.
[0073] The cellulose diacetate produc...
Embodiment 3
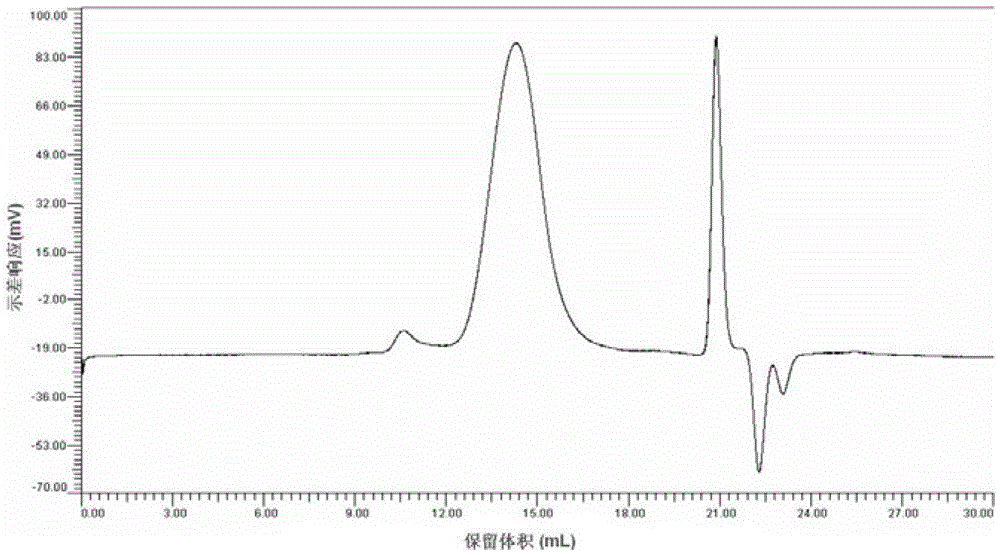
[0075]Put 1.248g of acetate-grade hardwood pulp dried at 105°C for 1 hour, together with 49.95g of trifluoroacetic acid and 12.48g of dichloromethane, into a three-neck flask with mechanical stirring, stir and infiltrate in an ice bath overnight, and then add 3.35 g (0.033mol) acetic anhydride to a transparent state, keep the reaction at 50°C for 3 hours, add a small amount of dichloromethane to complete the reaction, transfer to a rotary evaporator and treat at 50°C for 1 hour, and finally precipitate the obtained product in hot water, wash with water, Drying yielded 1.8 g of the product as a white powder. After analysis, the acetic acid value (AV) of this product is 53.80%. 1 The degree of substitution (DS) of the product was determined to be 2.33 by H-NMR. The product has been tested by GPC and FT-IR to prove that it does not contain sulfonate and trifluoroacetate groups. The product is soluble in pyridine, dimethyl sulfoxide and acetone.
PUM
| Property | Measurement | Unit |
|---|---|---|
| degree of substitution | aaaaa | aaaaa |
| degree of substitution | aaaaa | aaaaa |
| degree of substitution | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com