A method for preparing liquid crystal molecularly imprinted monolithic columns with chiral molecules as dopants
A technology of chiral molecules and liquid crystal molecules, applied in chemical instruments and methods, other chemical processes, etc., can solve the problems of inability to chromatographic stationary phase, difficult to resist high pressure of HPLC, etc., and achieve the effect of high imprinting effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
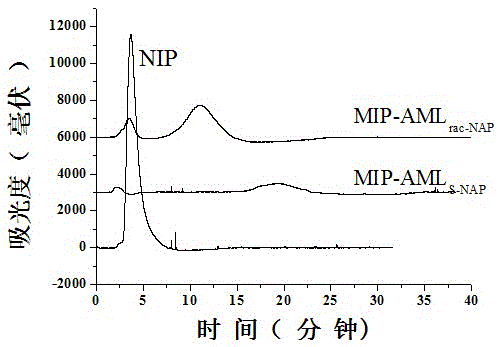
[0033] Preparation of liquid crystal low cross-linked amlodipine molecularly imprinted monolithic column induced by chiral dopant L-naproxen and evaluation of its imprinting effect.
[0034] A chiral dopant-induced liquid crystal low cross-linked amlodipine molecularly imprinted monolithic column was synthesized by surface imprinting method, and its retention performance was evaluated by connecting it to high performance liquid chromatography under appropriate chromatographic conditions. Synthetic reaction conditions and processing method are as follows:
[0035] Preparation of amlodipine as template molecularly imprinted monolithic column by surface imprinting method:
[0036]a. The initiator azobisisobutyronitrile with a mass fraction of 0.50% is dissolved in the porogen toluene with a mass fraction of 18.17% and dodecyl alcohol with a mass fraction of 51.70%, and then added trimethylol with a mass fraction of 29.63% propane trimethacrylate, ultrasonic 10min, pour the mixtu...
Embodiment 2
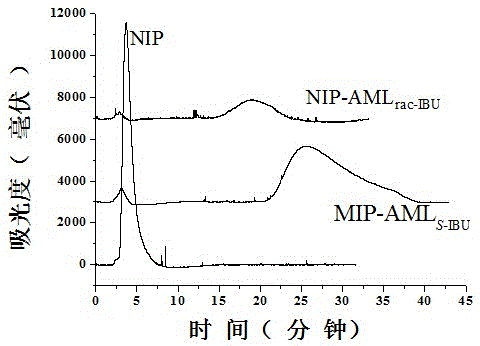
[0041] Preparation of liquid crystal low cross-linked amlodipine molecularly imprinted monolithic column induced by chiral dopant levoibuprofen and evaluation of its imprinting effect.
[0042] In order to demonstrate that the effect of chiral dopants on the overall imprinting effect of low-crosslinked amlodipine liquid crystal molecules is universal, we synthesized the addition of different chiral dopants (levo-ibuprofen) and the corresponding racemization Molecularly imprinted monolithic column of amlodipine with molecule as dopant. The specific operation steps are as follows:
[0043] a. The initiator azobisisobutyronitrile with a mass fraction of 0.50% is dissolved in the porogen toluene with a mass fraction of 18.17% and dodecyl alcohol with a mass fraction of 51.70%, and then added trimethylol with a mass fraction of 29.63% propane trimethacrylate, ultrasonic 10min, pour the mixture into a stainless steel column (1004.6mmI.D.), seal it up and let it stand vertically in ...
Embodiment 3
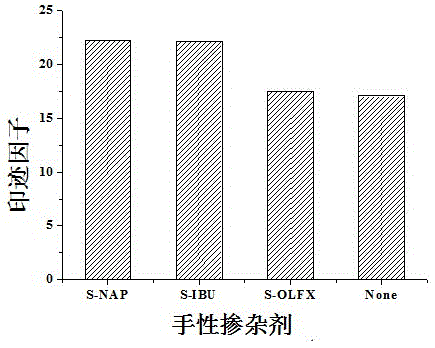
[0048] Preparation of monolithic columns for molecular imprinting of liquid crystals with low cross-linking amlodipine induced by chiral dopant levofloxacin and evaluation of their imprinting effects.
[0049] In order to further demonstrate the generality of the effect of chiral dopants on the imprinting effect of low-crosslinked amlodipine liquid crystal molecular monolithic imprinted columns, we also synthesized amlodipine molecularly imprinted monolithic columns added with levofloxacin dopant. The specific operation steps are as follows:
[0050] a. The initiator azobisisobutyronitrile with a mass fraction of 0.50% is dissolved in the porogen toluene with a mass fraction of 18.17% and dodecanol with a mass fraction of 51.7%, and then added trimethylol with a mass fraction of 29.63% propane trimethacrylate, ultrasonic 10min, pour the mixture into a stainless steel column (1004.6mmI.D.), seal it up and let it stand vertically in a constant temperature water bath at 48°C for ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com