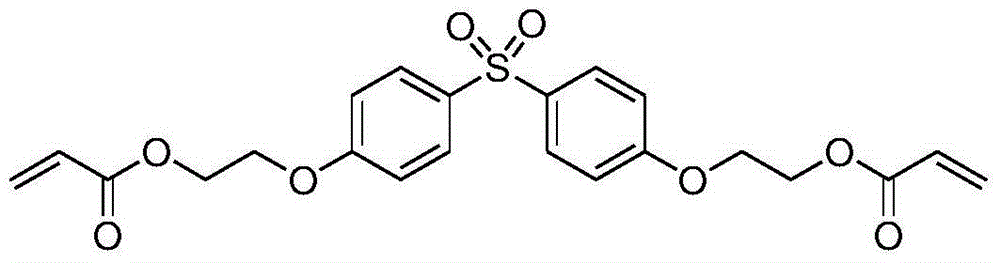
Ethoxy (2) bisphenol S diacrylate and preparation method thereof
A technology of diacrylate and ethoxylation, which is applied in chemical instruments and methods, preparation of organic compounds, organic chemistry, etc. It can solve the problems that small and medium-sized enterprises cannot afford, the catalyst regeneration process is complicated, and the catalytic efficiency is low, so as to achieve safety High, low cost, high catalytic efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
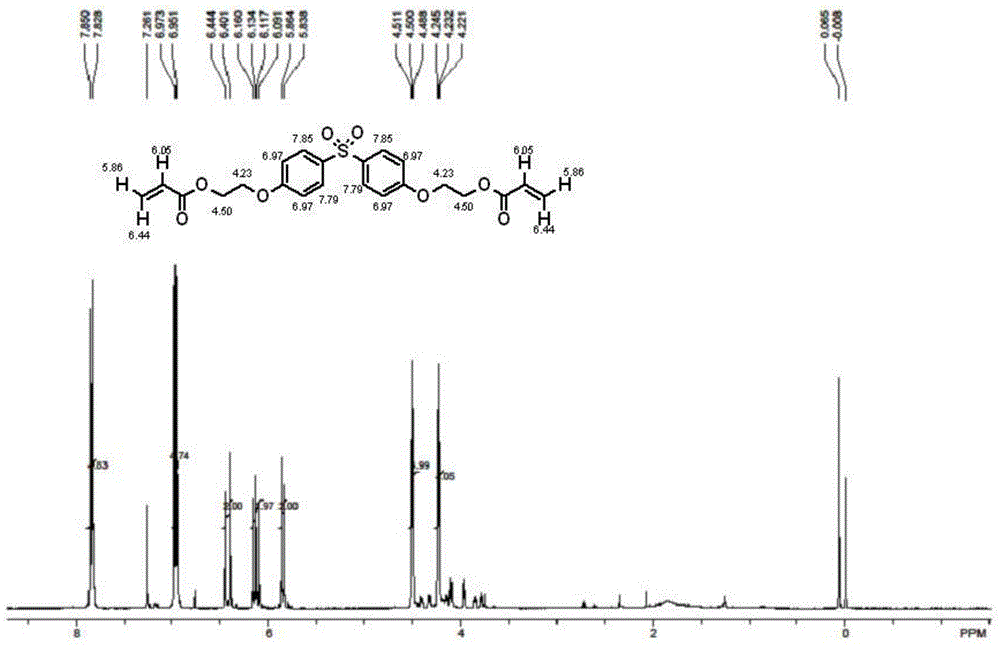
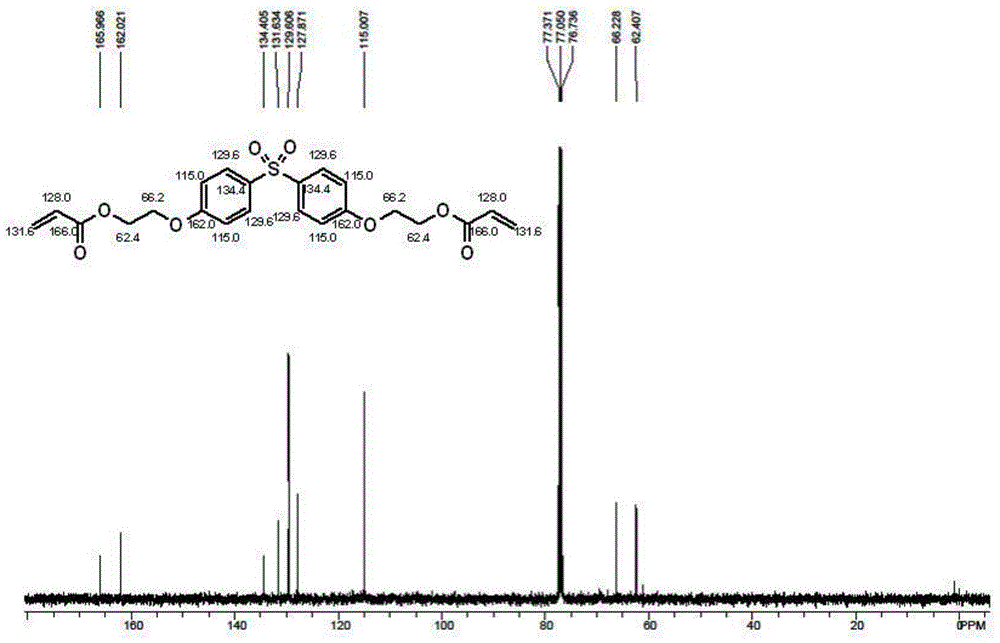
[0041]Add 19.0 grams of dihydroxyethyl bisphenol S (0.056mol, 0.112mol-OH, 1eq, 2eq-OH) and 12.1 grams of acrylic acid ( 0.16mol, 3.0eq, 1.5eq-OH), 46 grams of toluene, 0.54 grams of methanesulfonic acid (0.0056mol, 10eq%), 0.16 grams of p-hydroxyanisole (0.5wt%), nitrogen protection has been passed during the reaction , after reflux reaction at 110-115°C for 6 hours, a brownish-red liquid was obtained, and about 2.0 ml of water was generated. TLC analysis (exhibiting liquid: volume of ethyl acetate / volume of cyclohexane=1 / 1) has not observed dihydroxy Ethyl bisphenol S(R f =0.07) points; and only the product ethoxylated (2) bisphenol S diacrylate (R f =0.86), the reaction was stopped.
[0042] After the reaction, the reaction mixture was washed twice with 5% aqueous sodium hydroxide solution, then washed three times with distilled water, and finally dried with anhydrous magnesium sulfate, and then 0.03 p-hydroxyanisole, a polymerization inhibitor, was added to the dried sol...
Embodiment 2
[0044] Add 19.0 grams of dihydroxyethyl bisphenol S (0.056mol, 0.112mol-OH, 1eq, 2eq-OH) and 12.1 grams of acrylic acid ( 0.16mol, 3.0eq, 1.5eq-OH), 46 grams of toluene, 0.96 grams of p-toluenesulfonic acid (0.0056mol, 10eq%), 0.16 grams of p-hydroxyanisole (0.5wt%), nitrogen protection has been passed during the reaction , after reflux reaction at 110-115°C for 6.5 hours, a brownish-red liquid was obtained, and about 2.0 ml of water was generated. TLC analysis (exhibited liquid: volume of ethyl acetate / volume of cyclohexane=1 / 1) has not observed dihydroxy Ethyl bisphenol S(R f =0.07) points; and only the product ethoxylated (2) bisphenol S diacrylate (R f =0.86), the reaction was stopped.
[0045] After the reaction, the reaction mixture was washed twice with 5% aqueous sodium hydroxide solution, then washed three times with distilled water, and finally dried with anhydrous magnesium sulfate, and then 0.03 p-hydroxyanisole, a polymerization inhibitor, was added to the dried...
Embodiment 3
[0047] The technical scheme of embodiment 3 is identical with embodiment 1, only difference is as follows:
[0048] (1) The mol ratio of dihydroxyethyl bisphenol S and acrylic acid is 1:2.2;
[0049] (2) The consumption of solvent toluene is 0.8 times of the weight sum of dihydroxyethyl bisphenol S and acrylic acid;
[0050] (3) The consumption of p-toluenesulfonic acid is 2% of the molar weight of dihydroxyethyl bisphenol S;
[0051] (4) polymerization inhibitor is p-hydroxyanisole, and its consumption is 0.1% of the gross weight of dihydroxyethyl bisphenol S and acrylic acid;
[0052] (5) The esterification reaction time is 8 hours.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| refractive index | aaaaa | aaaaa |
| refractive index | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com