Preparation method of hexa(4-methoxyphenoxyl)cyclotriphosphazene
A technology of methoxyphenoxy and hexachlorocyclotriphosphazene, which is applied in the field of preparation of hexacyclotriphosphazene, can solve the problems of complex post-processing and long reaction time, and achieve easy control of reaction conditions and high yield , the effect of mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Add 12ml of acetone and 1.2g (30mmol) of sodium hydride (60%) into a 250ml round-bottomed flask, slowly add 15ml of acetone solution with 3.72g (30mmol) of p-hydroxyanisole dropwise, 800W ultrasonic for 10min, and place Add dropwise 22ml of acetone solution containing 1.74g (5mmol) hexachlorocyclotriphosphazene into a round-bottomed flask under ultrasonication. After the dropwise addition, ultrasonicate at 800W and 45°C for 1h, reflux for 11.5h, and distill under reduced pressure after the reaction is completed. Remove the solvent in the system to obtain a light yellow turbid viscous liquid, then add 30ml of dichloromethane dropwise to the viscous liquid, wash with deionized water until the water phase is neutral, separate the liquids and dry the organic phase with anhydrous magnesium sulfate for 5min, reduce Dichloromethane was evaporated under pressure to obtain hexa(4-methoxyphenoxy)cyclotriphosphazene with a yield of 89.3%.
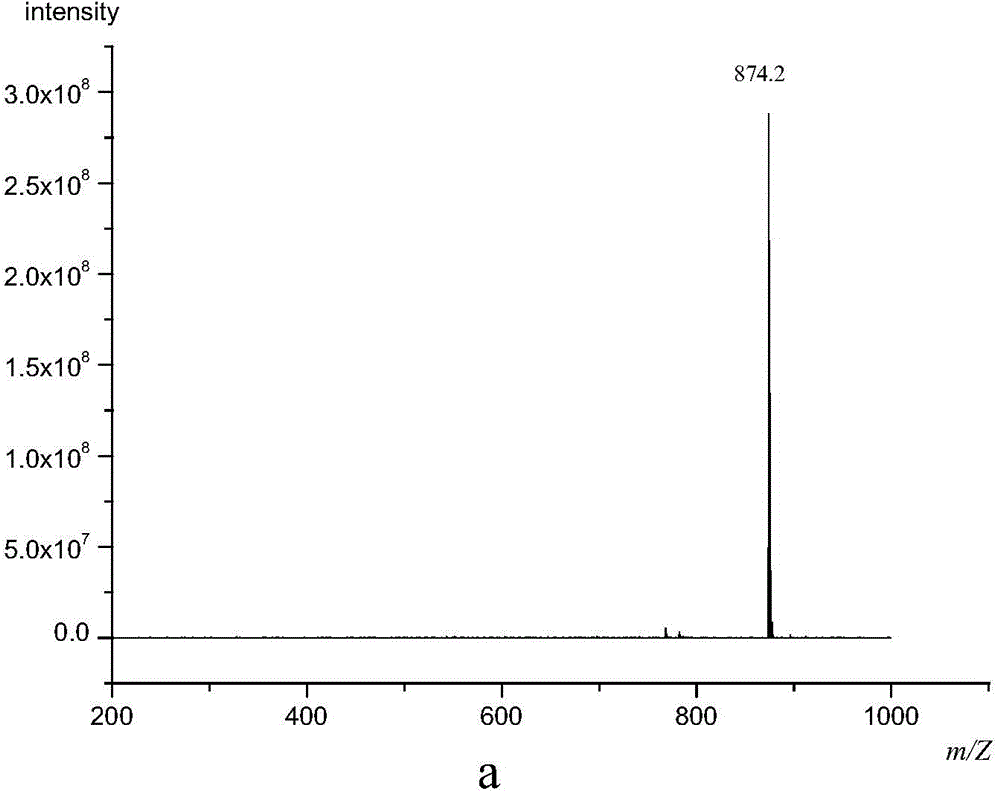
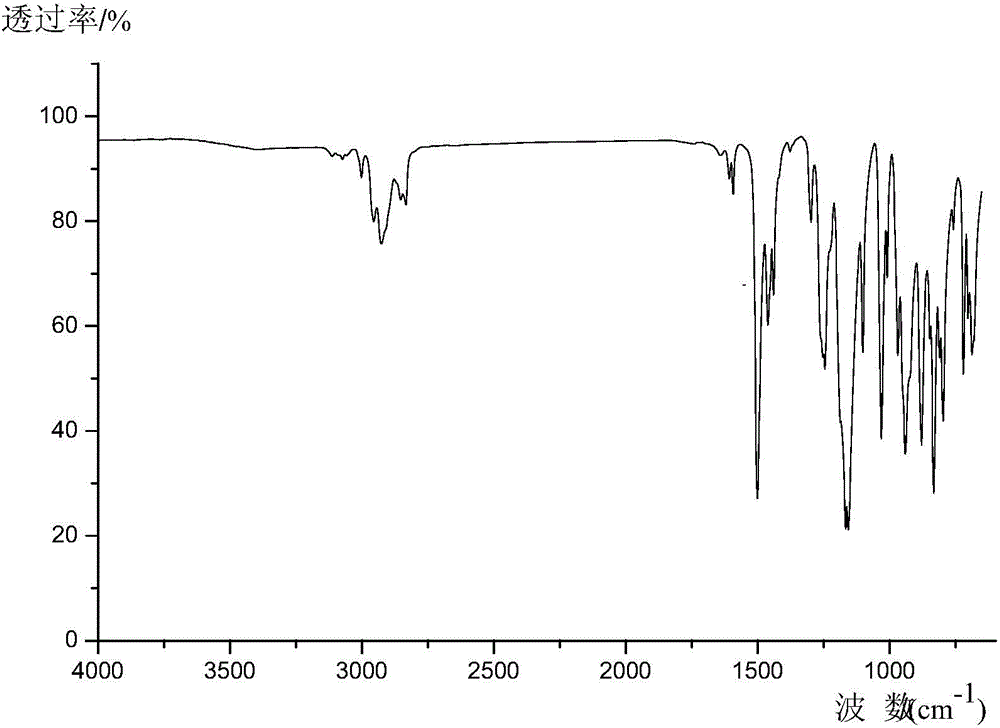
[0029] The molecular formula of hexa(4-m...
Embodiment 2
[0031] 17ml of tetrahydrofuran and 1.41g (35.25mmol) of sodium hydride (60%) were added to a 250ml round-bottomed flask, and 15ml of tetrahydrofuran solution containing 4.37g (35.25mmol) of p-hydroxyanisole was slowly added dropwise, followed by ultrasonication at 950W for 15min, and then Add dropwise 14ml of tetrahydrofuran solution containing 1.74g (5mmol) hexachlorocyclotriphosphazene to a round-bottomed flask placed in ultrasonication. After the addition is completed, ultrasonicate at 950W and 55°C for 2h, reflux for 12h, and depressurize after the reaction is completed. Distill the liquid after the reaction to obtain a light yellow turbid viscous liquid, then add 30ml of dichloromethane dropwise to the viscous liquid, wash the above liquid with deionized water to neutrality, separate the liquids to get the organic phase, dry it with anhydrous magnesium sulfate for 5min, reduce The solvent dichloromethane was evaporated under pressure to obtain hexa(4-methoxyphenoxy)cyclotr...
Embodiment 3
[0034] Add 20ml of acetone and 1.92g (48mmol) of sodium hydride (60%) into a 250ml round-bottomed flask, slowly add 20ml of tetrahydrofuran solution containing 4.96g (40mmol) of p-hydroxyanisole dropwise, 900W ultrasonic for 20min, and place Add dropwise 12ml of dioxane solution containing 1.74g (5mmol) hexachlorocyclotriphosphazene to a round-bottomed flask under ultrasonication. After the addition is completed, ultrasonicate at 850W and 40°C for 2.5h, and reflux for 11h. After the reaction is completed, Distill the reaction liquid under reduced pressure to obtain a light yellow turbid viscous liquid, then add 30ml of dichloromethane dropwise to the viscous liquid, wash the above liquid with deionized water until neutral, separate the liquids to take the organic phase, and dry it with anhydrous calcium chloride After 30 minutes, dichloromethane was evaporated under reduced pressure to obtain hexa(4-methoxyphenoxy)cyclotriphosphazene with a yield of 93.1%.
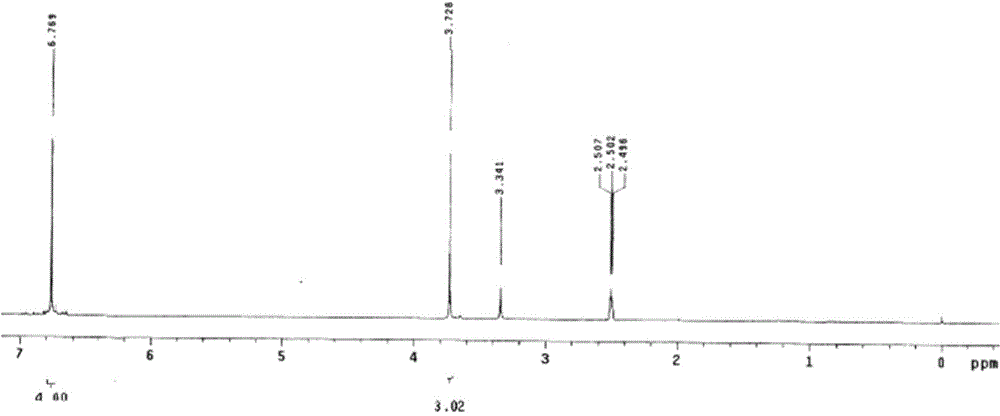
[0035] 1 H-NMR (D...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com