Trypsin mutant capable of improving enzyme activity and construction method thereof
A technology of trypsin and mutants, which is applied in the field of trypsin mutants and its construction, can solve the problems of low trypsin activity and low protein expression, and achieve the effects of improving extracellular secretion, high enzyme activity and simple fermentation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Embodiment 1 Contains the construction of the recombinant vector of trypsin mutant
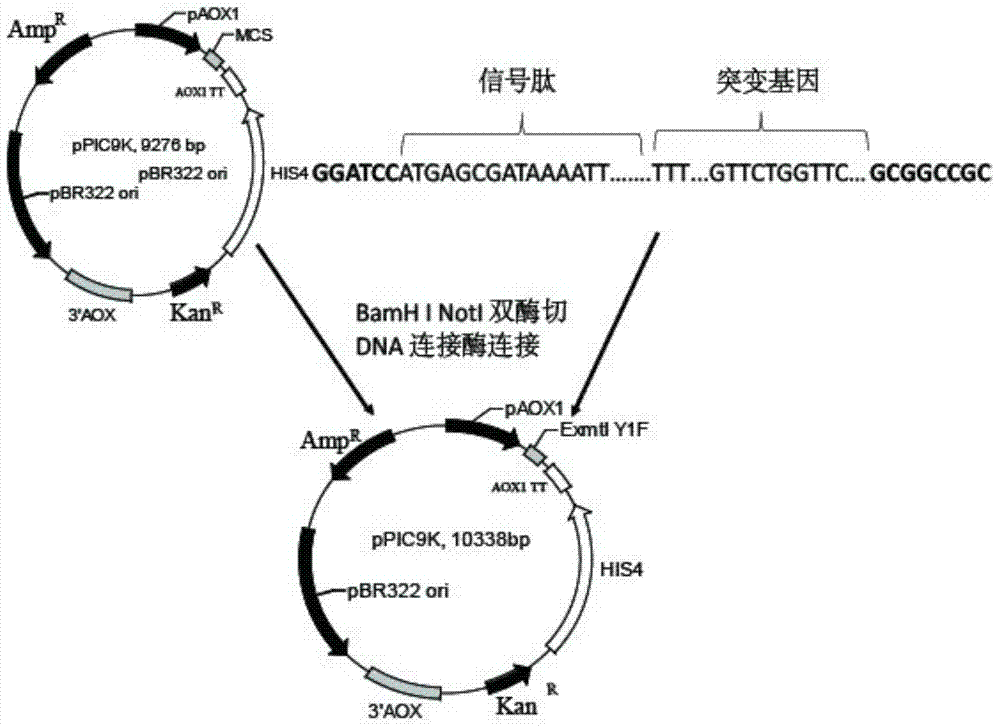
[0029] use figure 1 The methods shown were used to construct recombinant plasmid vectors.
[0030] (1) Obtaining the R123I mutant: using the sequence shown in SEQ ID NO.4 as a template, using F1primer (sequence shown in SEQ ID NO.5) and R1primer (sequence shown in SEQ ID NO.6) as primers, carry out The gene sequence of the mutant (R123I) in which arginine at position 123 of the coding amino acid sequence is mutated to isoleucine was obtained by PCR.
[0031] (2) The gene sequence of the mutant obtained in the above step is used as a template, and F2primer (sequence shown in SEQ ID NO.7) and R2primer (sequence shown in SEQ ID NO.8) are used as primers to carry out PCR to obtain the sequence as shown in The recombinant gene shown in SEQ ID NO.3 (ie, the sequence encoding the Y1F mutant). Connect the recombinant gene to the Sample T vector.
[0032] (3) The Sample T vector containing t...
Embodiment 2
[0033] Embodiment 2 produces mature trypsin yeast engineered bacterium construction
[0034] The recombinant plasmid pPIC9K-ExmtIY1F obtained in Example 1 was linearized with Sal I, and electroporated to transform pichiapastoris GS115 competent cells, the specific method is as follows:
[0035] 1) Inoculate pichiapastoris GS115 activated on YPD plate in 25mL / 250mL Erlenmeyer flask, and culture overnight at 30°C; inoculate 1% of the above culture solution into 50mL / 500mL Erlenmeyer flask, and the cultured bacteria concentration OD600 is 1.3-1.5;
[0036] 2) 5000r / min, centrifuge at 4°C for 10min to collect the bacteria, and suspend the cells with 50mL and 25mL sterile water respectively;
[0037] 3) Resuspend the above cells in 5mL of 1M sorbitol, centrifuge at 5000r / min, 4°C for 10min to collect the cells;
[0038] 4) Resuspend the above cells in 500 μL of 1M sorbitol, aliquot into 80 μL / 1.5mL EP tubes for electrotransformation of competent cells;
[0039] 5) Mix 20 μL of li...
Embodiment 3
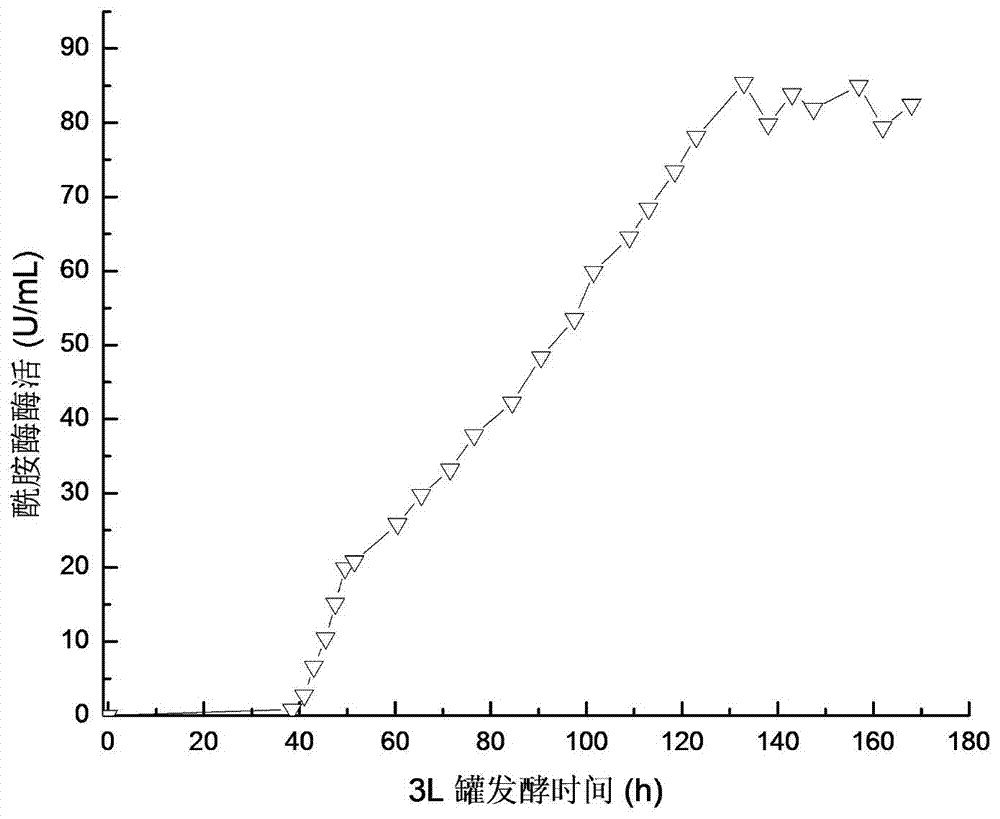
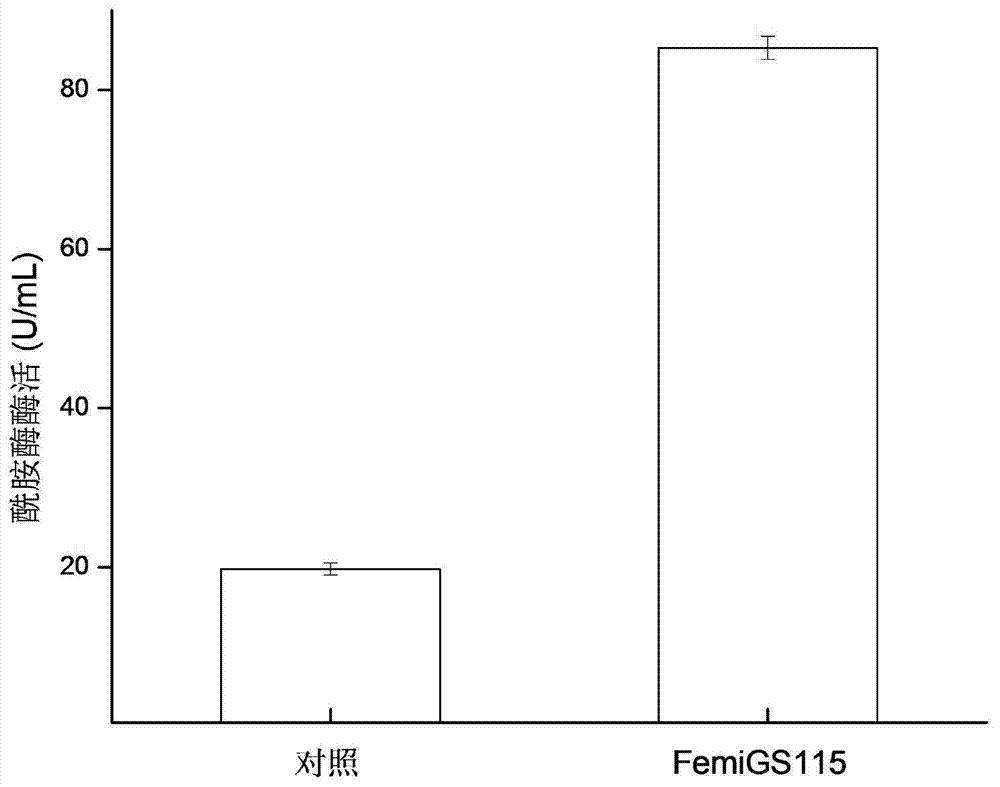
[0043] Embodiment 3 Recombinant Pichia pastoris 3L tank culture
[0044] The recombinant strain FemiGS115 constructed in Example 2 was used as a production strain and activated on a YPD plate. For seed liquid culture, inoculate 50mL / 250mL seed medium, and cultivate at 30°C and 220r / min for 24h. 10% was inoculated with 800mL / 3L fermentation medium, pH5.5, cultured in stages at 30°C: 0-17h, 500rmp / min culture, DO dropped from 100% to about 8%, and then rose to about 60%; 17-30h, The rotation speed was gradually increased to 1000rmp / min, and 50% glycerol was fed exponentially, DO began to drop to about 10%, and then rose to 70%; 30-144h, 1.8% (V / V) methanol was fed to induce trypsin production. Pichia pastoris expressing trypsin whose nucleotide sequence is shown in SEQ ID NO.4 was used as a control (ie, a strain without mutation).
[0045] Seed medium (g / L): 20 peptone, 10 yeast extract, 20 glucose.
[0046] Fermentation medium (g / L): glycerol 40; K 2 SO 4 18; KOH4.13; MgSO...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com