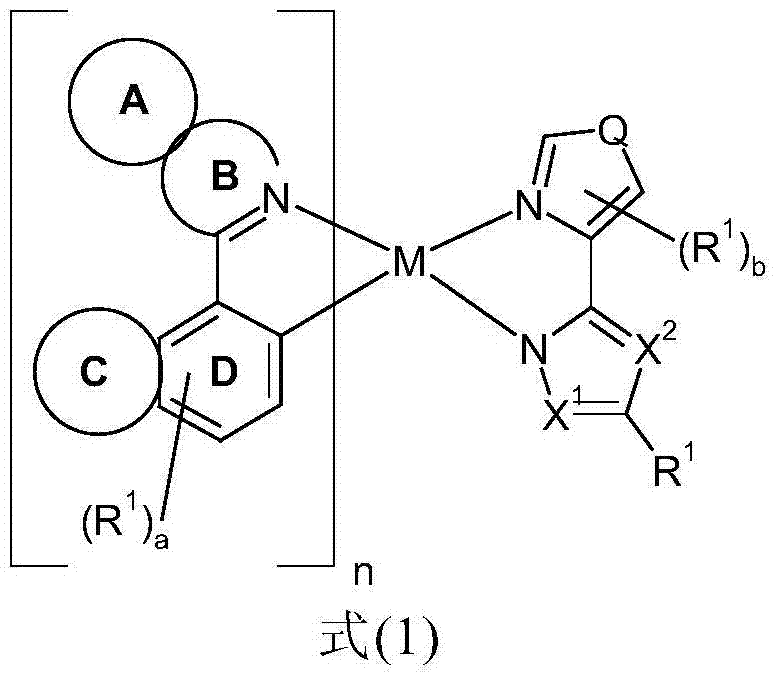
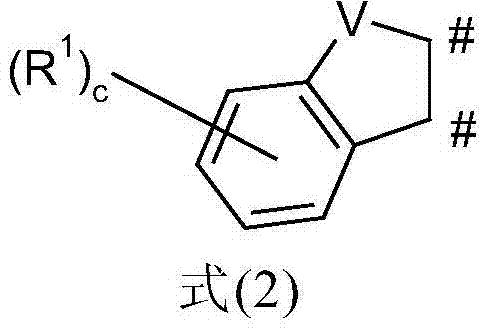
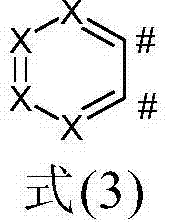Metal complexes comprising condensed heteroaromatic rings
A heteroaromatic ring and aromatic ring technology, applied in the field of new organometallic complexes, can solve the problems of rare emitters
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0214] Synthesis of 2-(Dibenzo[b,d]furan-4-yl)quinoline (dbfqH)
[0215]
[0216] To 2-chloroisoquinoline (1.73g, 10.5mmol) and Pd(PPh 3 ) 4 (403 mg, 0.349 mmol) in 1,2-dimethoxyethane (50 mL), add dibenzofuran-4-ylboronic acid (2.30 g, 10.9 mmol) in degassed ethanol (50 mL) solution, followed by the addition of 2.6M aqueous sodium carbonate (50 mL). The mixture was then heated at reflux for 19 hours under an inert atmosphere. After cooling, ethyl acetate (50 mL) and water (100 mL) were added, and insoluble materials were removed by filtration. The filtrate was worked up with standard aqueous work-up. The solvent was removed and the residue was purified by column chromatography to give a yellow solid. Recrystallization from chloroform / hexane gave the title compound dbfqH (2.62 g, 8.87 mmol) in 84% yield as a white crystalline solid.
Embodiment 2
[0218] Synthetic Ligands L1 to L44
[0219] Similar to the preparation of (dbfqh) according to Example 1, various analogous compounds such as compounds eg L1 to L44 can be prepared using the Suzuki coupling reaction. The general preparation method is as follows:
[0220] To the halogen compound (10.5mmol; educt 2) and Pd(PPh 3 ) 4 (0.35mmol, 1 / 30 equiv) in 1,2-dimethoxyethane (50mL) was added a solution of boric acid (11mmol; educt 1) in degassed ethanol (50mL), followed by 2.6M aqueous sodium carbonate solution (50 mL). Then, the mixture was heated at reflux for 20 hours under an inert atmosphere. After cooling, ethyl acetate (50 mL) and water (100 mL) were added, and insoluble materials were removed by filtration. The filtrate was worked up with standard aqueous work-up. The solvent was removed and the residue was purified by column chromatography. Recrystallization from chloroform / hexane afforded the compound as a white crystalline solid in about 65 to 90% yield.
...
Embodiment 3
[0231] Synthesis of 2-(3-(trifluoromethyl)-1H-pyrazol-5-yl)pyridine (fppzH) and 2-(3-(perfluorobutyl)-1H-pyrazol-5-yl)pyridine ( hppzH)
[0232]
[0233] The concentrations used in the reactions are provided for both fppzH and hppzH in the description below.
[0234] NaOEt (in case of fppzH: 1.82 g, 26.8 mmol; in case of hppzH: 1.23 g, 18.0 mmol) was dispersed in 50 mL of dry tetrahydrofuran (THF). To this solution was slowly added ethyl trifluoroacetate (3.2 mL, 26.8 mmol) for fppzH or ethyl nonafluoropentanoate (3.0 mL, 15.1 mmol) for hppzH at 0 °C . The mixture was stirred at 0° C. for 2 hours, followed by the addition of 2-acetylpyridine (in case of fppzH: 2.0 mL, 17.9 mmol; in case of hppzH: 1.87 mL, 16.7 mmol). The reaction was then heated to 50 °C for 4 hours. Removal of the solvent gave a brown oily residue which was dispersed in deionized water (50 mL). with 2N HCl (水 溶液) The mixture was neutralized (pH = 4), followed by extraction with ethyl acetate (3 x 50 m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com