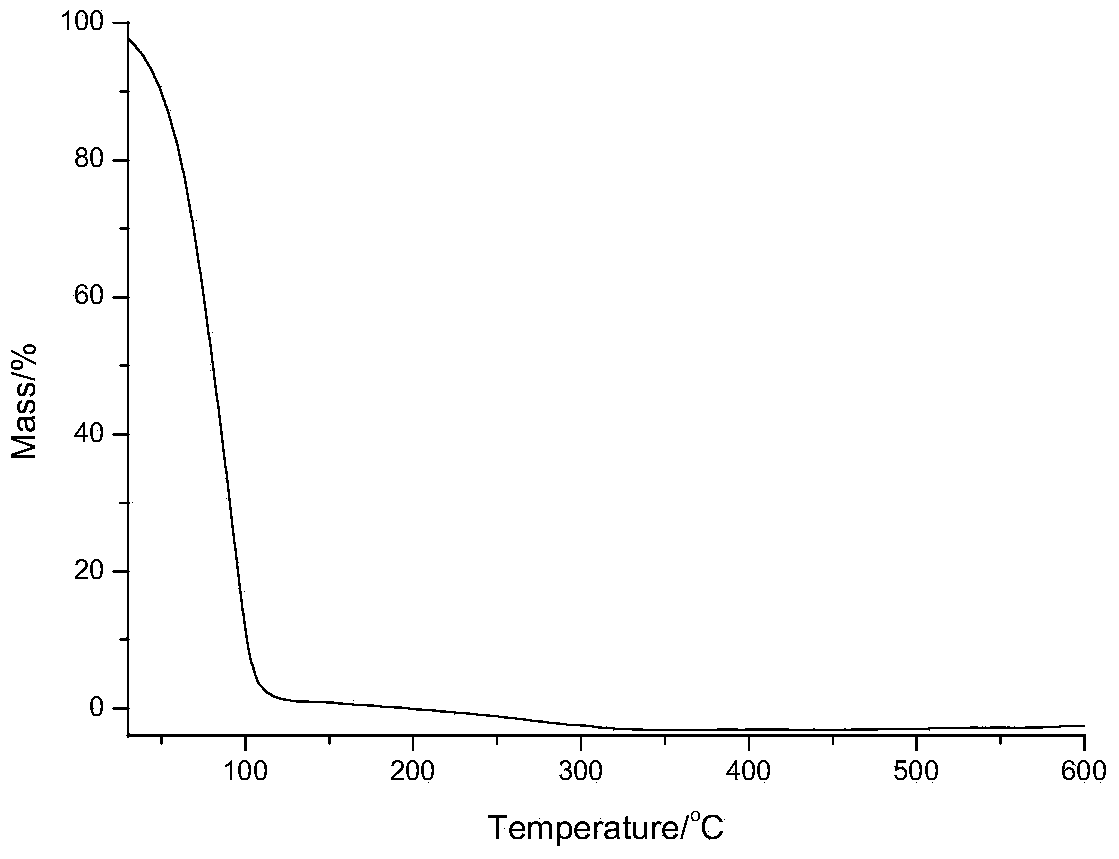
Compound containing N amidino-silicon and application thereof
A technology of silicon compound and amidine group, which is applied in the field of microelectronic materials, can solve the problems of low volatility, danger, and easy volatility, and achieve the effects of simple and easy processing, reduced synthesis cost, and mild synthesis conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
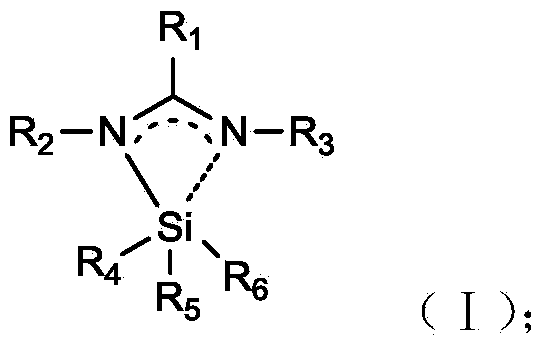
[0038] The preparation method of the N-amidino-containing silicon compound of the present invention is carried out according to the reaction of formula (I):
[0039]
[0040] where R 1 from C 1 ~C 10 Alkyl, C 2 ~C 10 Alkenyl, C 2 ~C 10 Alkynyl, C 3 ~C 10 Cycloalkyl, C 6 ~C 17 Aryl, —N(SiMe 3 ) 2 or—N(R 5 R 6 ), where R 2 , R 3 independent from C 1 ~C 10 Alkyl, C 2 ~C 10 Alkenyl, C 2 ~C 10 Alkynyl, C 3 ~C 10 Cycloalkyl, C 6 ~C 17 Aryl, where R 4 , R 5 , R 6 independently selected from hydrogen atom, halogen atom (F, Cl, Br, I), C 1 ~C 10 Alkyl, C 2 ~C 10 Alkenyl, C 2 ~C 10 Alkynyl, C 3 ~C 10 Cycloalkyl, C 6 ~C 17 Aryl, —Si(R 2 R 3 R 4 ), or—N(R 7 R 8 ), where R 5 , R 6 independently selected from hydrogen atom, C 1 ~C 10 Alkyl, C 2 ~C 10 Alkenyl, C 2 ~C 10 Alkynyl, C 3 ~C 10 Cycloalkyl, C 6 ~C 17 Aryl, where R 7 , R 8 independently selected from hydrogen atom, C 1 ~C 10 Alkyl, C 2 ~C 10 Alkenyl, C 2 ~C 10 Alkyny...
Embodiment 1
[0043] Embodiment one: a kind of preparation method that contains N-amidino silicon compound precursor body, comprises the following steps:
[0044] (1) will dissolved in n-hexane solvent, The mass ratio to n-hexane solvent is 1:10, and LiNiPr is added under the condition of stirring at -78°C 2 in ether solution, with LiNiPr 2 The molar ratio is 1:1, LiNiPr 2 The concentration of the diethyl ether solution was 1.0M, and the stirring speed was 800 rpm; after returning to room temperature, the stirring reaction was continued for 0.5 hours to obtain a reaction mixture;
[0045] (2) filter the reaction mixture obtained in step (1), collect the filter residue, obtain lithium salt solid, mix lithium salt solid with normal hexane, the mass ratio of lithium salt solid and normal hexane is 1:10, obtain the normal hexane of lithium salt alkane solution;
[0046] (3) According to lithium salt and SiH at 0°C 2Cl 2 The molar ratio is 2:1, the n-hexane solution of lithium salt is...
Embodiment 2
[0048] Embodiment two: a kind of preparation method that contains N-amidino silicon compound precursor body, comprises the following steps:
[0049] (1) will dissolved in toluene solvent, The mass ratio of toluene solvent is 1:15, and it is added to the n-hexane solution of tert-butyllithium under the condition of keeping stirring at -39°C, The molar ratio to tert-butyllithium is 1:1.1, the concentration of tert-butyllithium in n-hexane solution is 1.5M, and the stirring speed is 1500 rpm; after returning to room temperature, continue to stir and react for 1.5 hours to obtain a reaction mixture;
[0050] (2) filter the reaction mixture obtained in step (1), collect the filter residue, obtain lithium salt solid, mix lithium salt solid with toluene, the mass ratio of lithium salt solid and toluene is 1:20, obtain the toluene solution of lithium salt;
[0051] (3) According to lithium salt and SiF at -78°C 4 The molar ratio is 1:1, the toluene solution of lithium salt is ad...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com