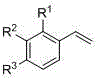
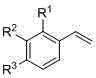
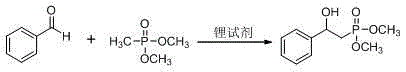Preparation method of beta-hydroxyphosphonate derivatives
A technology of hydroxyphosphonate and derivatives, which is applied in the field of preparation of β-hydroxyphosphonate derivatives, can solve the problems of poor substrate applicability, harsh reaction conditions, and high cost, and achieve stable raw materials, simplified operation steps, and high conversion rate effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Example 1: Synthesis of dimethyl 2-hydroxyl-2-phenylethylphosphonate
[0039] Using styrene and dimethyl phosphite as raw materials, the reaction formula and experimental steps are as follows:
[0040]
[0041] (1) Add styrene (0.11 g, 1 mmol), dimethyl phosphinate (0.11 g, 1 mmol), manganese acetate (0.41 g, 1.5 mmol) and 10 mL of acetic acid into the reaction flask, and react at 30°C Carry out, TLC follow-up reaction until the end;
[0042] (2) The reaction solution was concentrated to obtain a residue, which was separated by column chromatography (petroleum ether: ethyl acetate = 10:1) to obtain dimethyl 2-hydroxy-2-phenylethylphosphonate (yield 65%);
[0043] (3) Add dimethyl 2-hydroxy-2-phenylethylphosphonate (0.11 g, 0.5 mmol), ammonia water (0.12 g, 4.0 mmol) and 10 mL of acetonitrile obtained in (2) into the reaction flask, and react Carried out at 60°C, TLC followed the reaction until the end;
[0044] (4) The reaction solution was concentrated to remove ...
Embodiment 2
[0047] Embodiment two: the synthesis of diethyl 2-hydroxyl-2-phenylethylphosphonate
[0048] With styrene and diethyl phosphite as raw materials, its reaction formula and experimental steps are as follows:
[0049]
[0050] (1) Add styrene (0.1 g, 1 mmol), diethyl phosphite (0.20 g, 1.5 mmol), manganese acetate (0.41 g, 1.5 mmol) and 10 mL of acetonitrile into the reaction flask, and react at 40°C Carry out, TLC follow-up reaction until the end;
[0051] (2) The reaction solution was concentrated to obtain a residue, which was separated by column chromatography (petroleum ether: ethyl acetate = 10:1) to obtain diethyl 2-hydroxy-2-phenylethylphosphonate (yield 75%);
[0052] (3) Add diethyl 2-hydroxy-2-phenylethylphosphonate (0.14 g, 0.5 mmol) obtained in (2), ammonia water (0.12 g, 4.0 mmol) and 10 mL of acetonitrile into the reaction flask, and react Carried out at 60°C, TLC followed the reaction until the end;
[0053] (4) The reaction solution was concentrated to remo...
Embodiment 3
[0055] Embodiment three: two Synthesis of Isopropyl 2-Hydroxy-2-Phenylethyl Phosphonate
[0056] Taking styrene and diisopropyl phosphite as raw materials, its reaction formula and experimental steps are as follows:
[0057]
[0058] (1) Add styrene (0.1 g, 1 mmol), diisopropyl phosphite (0.25 g, 1.5 mmol), manganese acetate (0.55 g, 2 mmol) and 10 mL of dichloromethane into the reaction flask, and react Carried out at 30°C, followed by TLC until the end of the reaction;
[0059] (2) The reaction solution was concentrated to obtain a residue, which was separated by column chromatography (petroleum ether: ethyl acetate = 10:1) to obtain two Isopropyl 2-hydroxy-2-phenylethylphosphonate (74% yield);
[0060] two The analytical data for isopropyl 2-hydroxy-2-phenylethylphosphonate are as follows: 1 H NMR (300 MHz, CDCl 3 ): δ 7.41-7.27 (m, 5H), 4.73 (s, 1H), 4.75-4.70 (m, 2H), 4.48-4.38 (m, 1H), 2.23-2.13 (m, 2H), 1.27 (m, 12H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com