Fullerene polyazide glycidyl ether and its preparation method and application
A technology of glycidyl ether and fullerene, applied in the field of energetic combustion catalysts and its preparation, to achieve the effects of increased combustion speed, easy separation and purification, and simple preparation methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
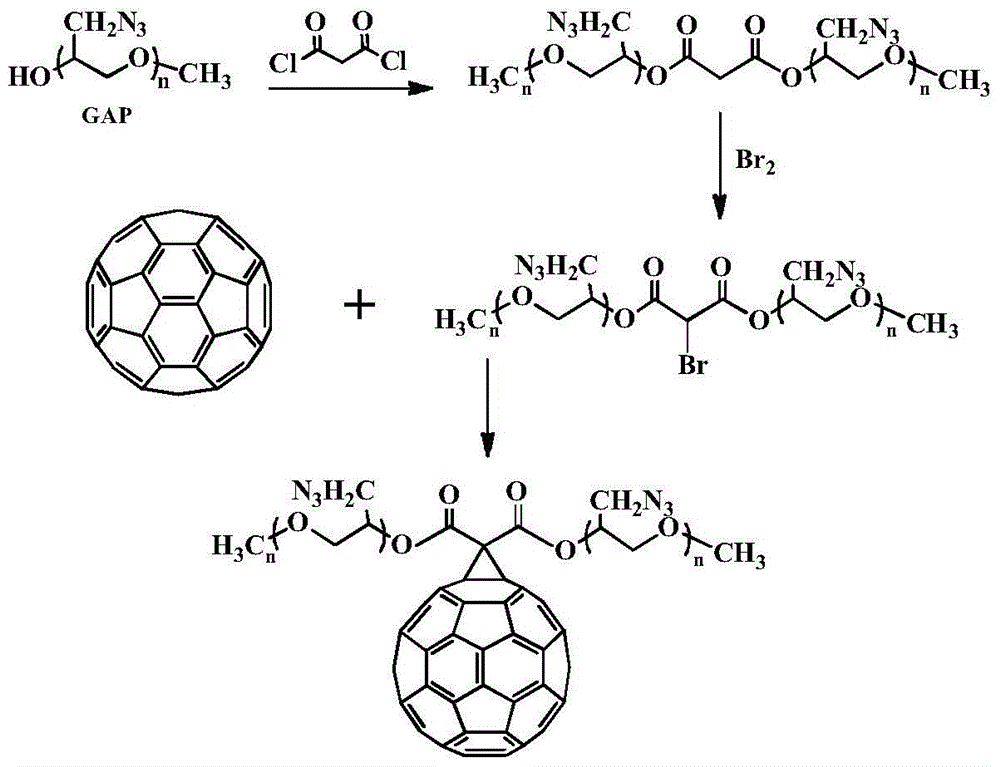
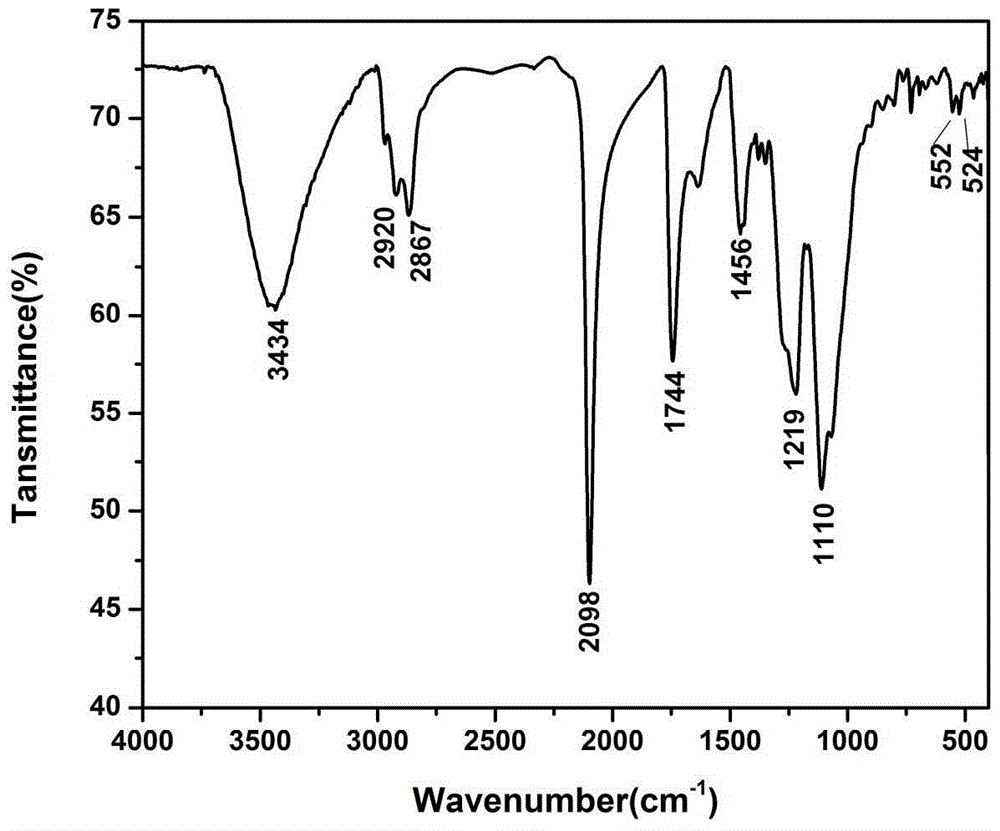
[0034] Example 1: (1) Put 10.00g of monomethyl polyazide glycidyl ether, 0.77mL of acid-binding agent N,N-dimethylformamide and 100mL of solvent dichloromethane into the reactor, at 0°C After diluting 1.87g of malonyl chloride with 30mL of dichloromethane, it was slowly dropped into the solution, and reacted at room temperature for 11h. The reaction solution was washed with water to a pH of about 7.0, dried and then evaporated to remove the solvent to obtain 9.4 g of malonic acid dimethyl polyazide glycidyl ether.
[0035] (2) Add 9.4g of the above-mentioned dimethyl malonate glycidyl azide and 150mL of dichloromethane in the reaction flask, and drop 1.93g of bromine into the reaction solution dropwise at room temperature until the reaction solution does not Fading, and then continue to stir the reaction 7h. After the reaction was completed, it was washed three times with saturated sodium bromide solution and distilled water successively, dried with anhydrous sodium sulfate, ...
Embodiment 2
[0042] Embodiment 2: Preparation of fullerene polyazide glycidyl ether
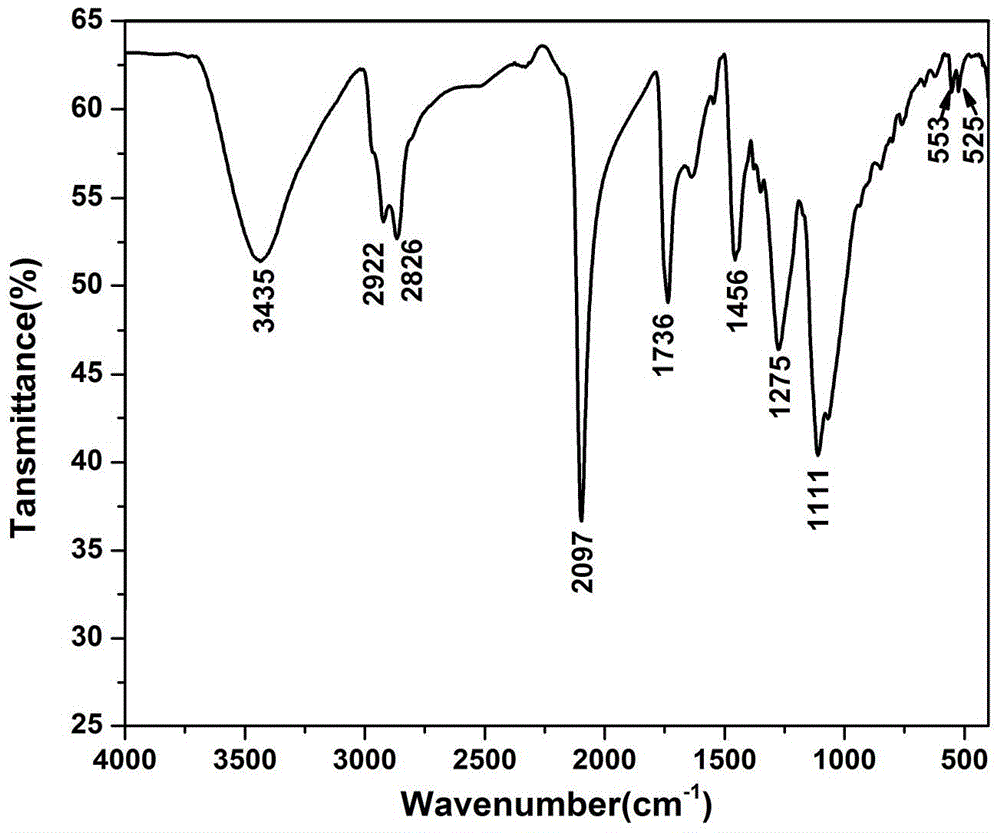
[0043] (1) Under nitrogen or argon atmosphere, put 10.00g of monomethyl polyazide glycidyl ether, 1.0mL of acid-binding agent triethylamine and 100mL of solvent methylene chloride into the reactor, and put 3.75g of Malonyl chloride was diluted with 40 mL of dichloromethane and slowly dropped into the solution, and reacted at room temperature for 10 h. The reaction solution was washed with water to a pH of about 7.0, dried and then evaporated to remove the solvent to obtain 10.8 g of malonate dimonomethyl polyazide glycidyl ether.
[0044] (2) Add 9.2g of the above-mentioned dimethyl malonate glycidyl azide and 150mL of dichloromethane in the reaction flask, and drop 3.8g of bromine into the reaction solution dropwise at room temperature until the reaction solution does not Fading, and then continue to stir the reaction 7h. After the reaction was completed, it was washed three times with saturated sodium...
Embodiment 3
[0051] Embodiment 3: Preparation of fullerene polyazide glycidyl ether
[0052] (1) Put 10.00g of monomethyl polyazide glycidyl ether, 0.60mL of acid-binding agent pyridine and 100mL of solvent methylene chloride into the reactor, and dilute 2.72g of malonyl chloride with 30mL of methylene chloride at 0°C Then it was slowly dropped into the solution and reacted at room temperature for 11h. The reaction solution was washed with water to a pH of about 7.0, and after drying, the solvent was removed by rotary evaporation to obtain 9.4 g of dimonomethyl polyazide glycidyl malonate
[0053] (2) Add 8.8g of the above-mentioned dimethyl malonate glycidyl azide ether and 150mL of dichloromethane into the reaction flask, add 3.2g of N-bromosuccinimide at room temperature, and then stir at 60°C Reaction 4h. After the reaction was completed, it was washed three times with saturated sodium bromide solution and distilled water successively, dried with anhydrous sodium sulfate, filtered, a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com