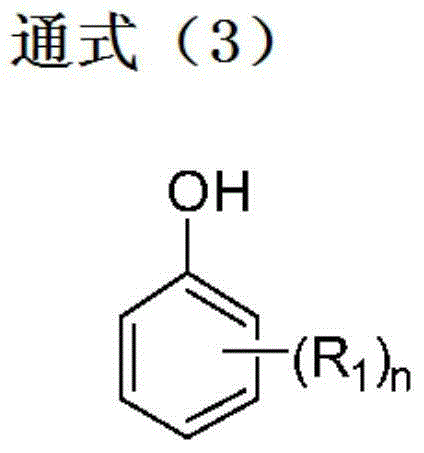
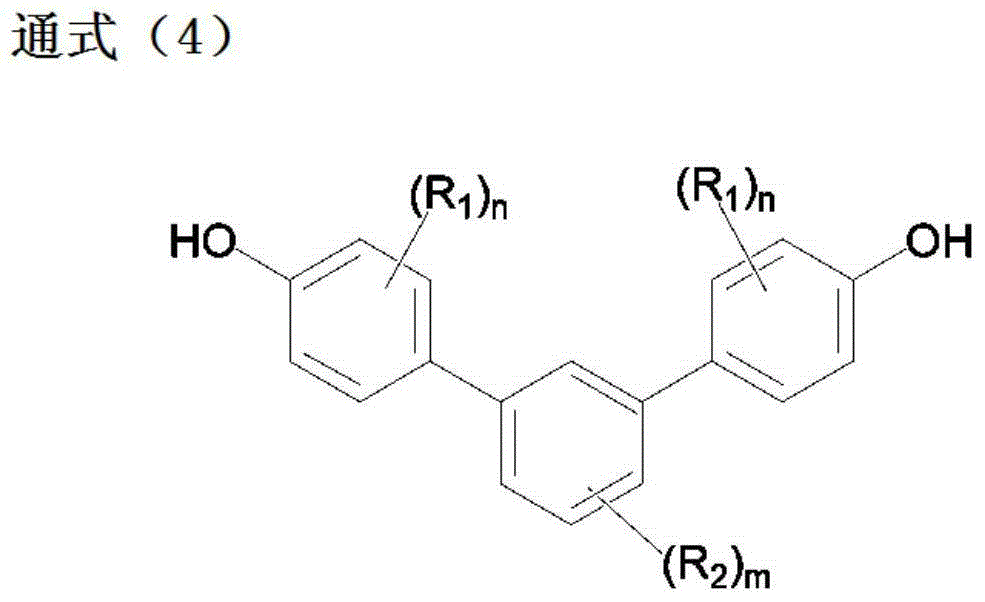
Method for producing 4,4''-dihydroxy-m-terphenyls
A technology of m-terphenyl and its manufacturing method, which is applied in 4 fields and can solve problems such as difficult industrial implementation, high manufacturing cost, and no record
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0174] [Synthesis of 4,4"-dihydroxy-m-terphenyl]
[0175] Step (A): (Synthesis of 1,1,3-tris(4-hydroxyphenyl)cyclohexane)
[0176] Put 1412g of phenol, 78.2g of 35% hydrochloric acid, 15.2g of dodecyl mercaptan, and 144g of methanol into a four-necked flask with a capacity of 3 liters, and keep the liquid temperature at 30-32°C under a nitrogen atmosphere, while adding 144g of 2- Cyclohexen-1-one was used for 10 hours, and after the dropwise addition was terminated, the mixture was stirred at 30° C. for 46 hours. After the reaction was terminated, an aqueous sodium hydroxide solution was added for neutralization, and then the temperature was raised to distill off methanol. Then, water and methyl isobutyl ketone were added to the oil layer obtained by separating and removing the water layer, stirred and washed with water, and the water layer was separated and removed. Phenol which had not reacted with methyl isobutyl ketone was distilled off from the obtained oil layer under ...
Embodiment 2
[0207] [Synthesis of 4,4"-dihydroxy-m-terphenyl]
[0208] 90g of 1,1,3-tri(4-hydroxyphenyl)cyclohexane and 45g of tetraethylene glycol, 45g of methyl alcohol, 6.3g of 16% sodium hydroxide aqueous solution obtained in the operation (A) of embodiment 1 were dropped into four In the mouth bottle, the pressure was reduced to 10kPa while stirring, and at the same time, the temperature of the liquid was raised to 170°C, and the phenol generated at the same temperature was further distilled off while stirring for 9 hours to carry out the reaction. After completion of the reaction, after cooling, acetic acid was added to neutralize, and then water and methyl isobutyl ketone were added to stir and wash with water [process (B)].
[0209] After separating the water layer, 1,3-bis(4-hydroxyphenyl)-1-cyclohexene and 1,5-bis(4-hydroxyphenyl)-1-cyclohexene in the oil layer were separated by concentration etc. Adjust the concentration of the mixture to 17%. 79.6 g of this solution, 14.8 g o...
Embodiment 3
[0212] [Synthesis of 3,3"-dimethyl-4,4"-dihydroxy-m-terphenyl]
[0213] Step (A): (Synthesis of 1,1,3-tris(3-methyl-4-hydroxyphenyl)cyclohexane)
[0214]Put 1513.4g of o-cresol, 73g of 35% hydrochloric acid, 14.2g of dodecyl mercaptan, and 134.4g of methanol into a four-necked flask with a capacity of 3 liters. 134.5 g of 2-cyclohexen-1-one was added for 3.5 hours, and after the dropwise addition was terminated, the mixture was stirred at 30 to 32° C. for 22 hours. After the reaction was terminated, an aqueous sodium hydroxide solution was added for neutralization, and then the temperature was raised to distill off methanol. Thereafter, methyl isobutyl ketone and water were added to the oil layer obtained by separating and removing the water layer, stirred and washed with water, and the water layer was separated and removed. From the obtained oil layer, o-cresol which had not reacted with methyl isobutyl ketone was distilled off under reduced pressure. After the residue was...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com