Aspartic acid-lysine copolymer and synthetic method thereof
A technology of lysine copolymer and aspartic acid, applied in chemical instruments and methods, water/sludge/sewage treatment, descaling and water softening, etc., can solve unstable product performance, slow reaction speed, production Low yield and other problems, to achieve the effect of stable product performance, fast reaction speed and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach 1
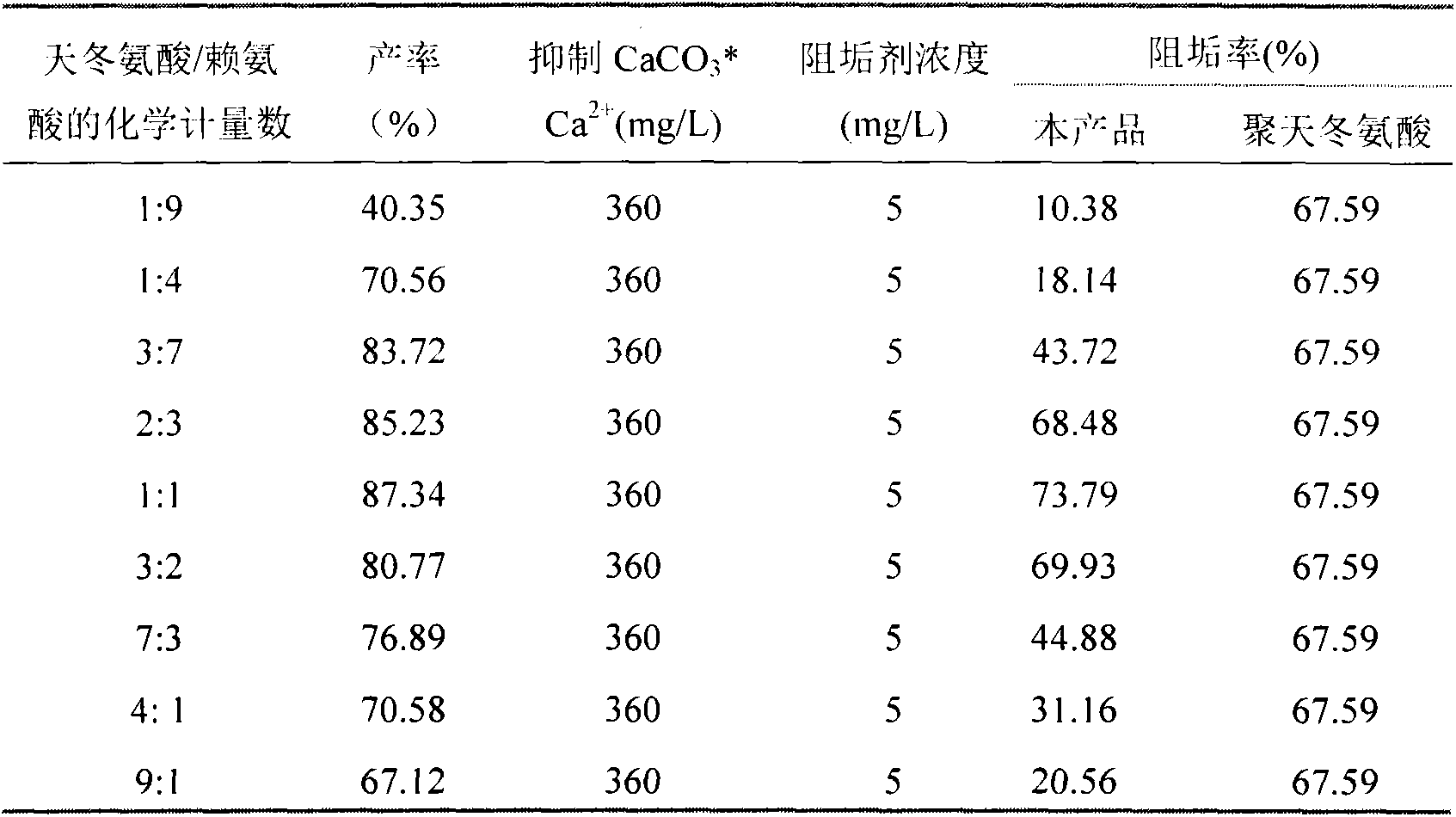
[0009] Specific embodiment one: the structural formula of the aspartic acid-lysine copolymer of the present embodiment is The range of x / (x+y) is 0.1-1, and the range of x+y is 3-100. The optimum range of x / (x+y) in this embodiment is 0.3-0.7, and the optimum range of x+y is 6-80.
specific Embodiment approach 2
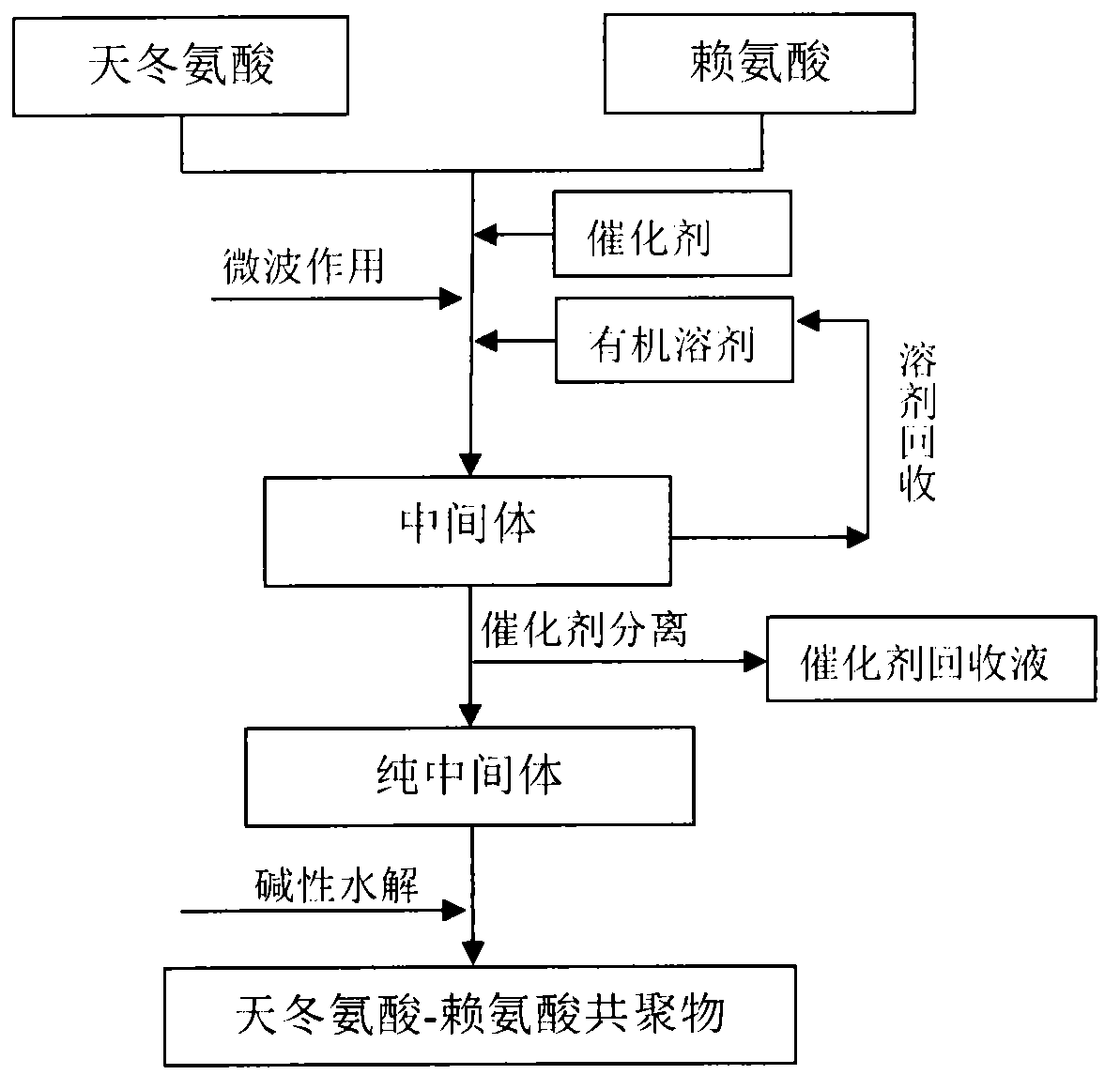
[0010] Specific embodiment two: this embodiment synthesizes aspartic acid-lysine copolymer according to the following steps:
[0011] Using aspartic acid and lysine as raw materials, using microwave technology, under the condition of microwave frequency of 915±50MHz or 2450±50MHz and microwave power of 400-10000W, a small amount of catalyst and organic solvent are added to the reactant, The intermediate of aspartic acid-lysine copolymer can be obtained by radiation reaction for 1 to 30 minutes. After the intermediate is washed and separated by catalyst, it can be hydrolyzed under alkaline conditions to obtain aspartic acid-lysine copolymer. (or polyaspartic acid derivatives).
[0012] In the synthesis process, the reaction time will be shortened with the increase of microwave power. Too short reaction time is not conducive to uniform reaction, so a stirring device is added, and the reaction time is not less than 4min; too high power will lead to dark color of the reaction prod...
specific Embodiment approach 4
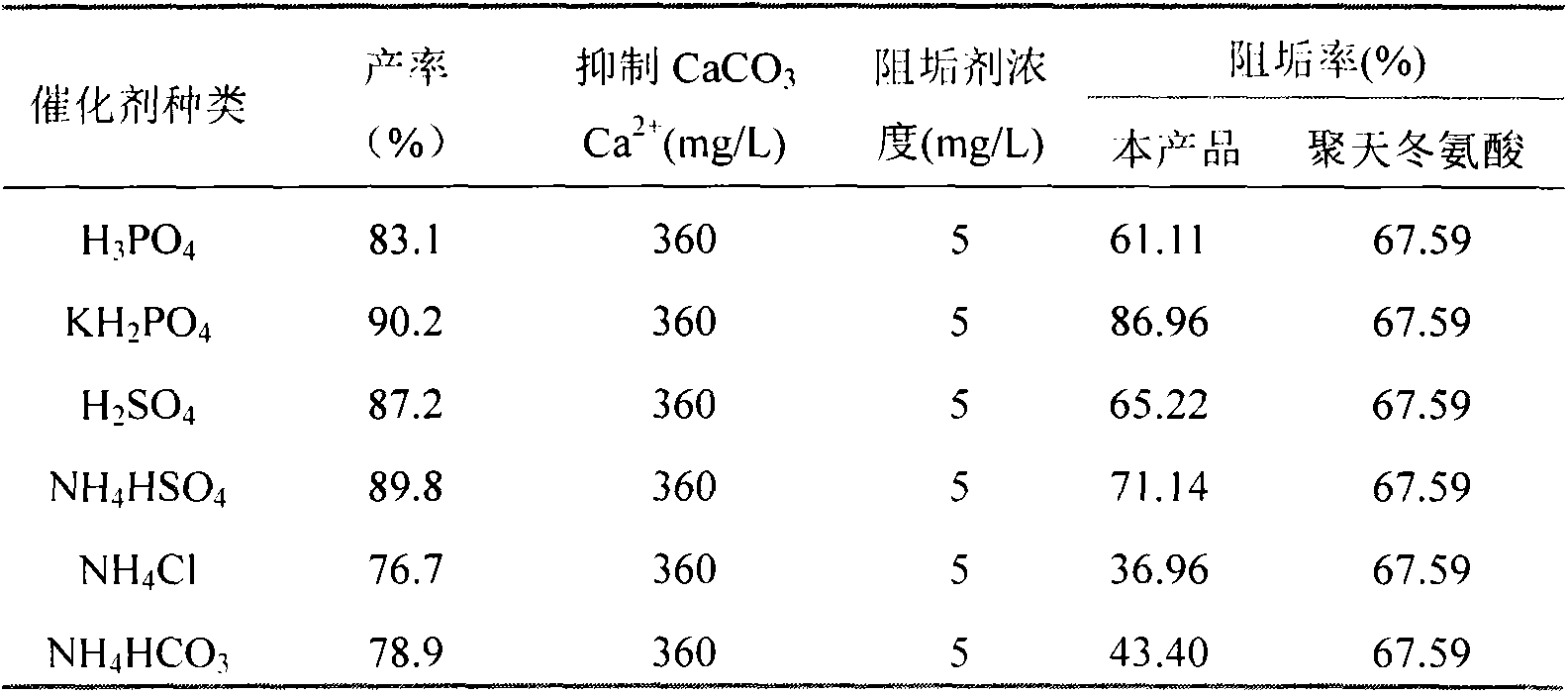
[0017] Specific embodiment four: aspartic acid and lysine respectively get 0.10mol, add three-neck round-bottomed flask; According to the kind of catalyst selected in Table 2, the ratio of catalyst and raw material stoichiometric number is 0.05; Add 16mL propylene carbonate, The reaction time was 8 minutes under microwave radiation, and the microwave power was 1200W. A series of brown and fluffy products were obtained. Add 120mL of pure water to rinse the product and separate the catalyst, add 42mL of 3mol / L NaOH to dissolve the product, adjust the pH of the solution to neutral, and filter , adding excess ethanol to the filtrate for dehydration, collecting the precipitate and vacuum drying at 70°C to obtain aspartic acid-lysine copolymer.
[0018] Table 2 The impact of the type of catalyst on the copolymer yield and scale inhibition performance
[0019]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com