Novel crystal form of p-aminosalicylic acid as well as preparation method and applications thereof
A technology of p-aminosalicylic acid and crystal form, applied in the field of medicinal chemistry, can solve the problems of high manufacturing cost and use cost, unstable aminosalicylic acid, etc., achieve high manufacturing cost and use cost, easy processing, The effect of short process steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
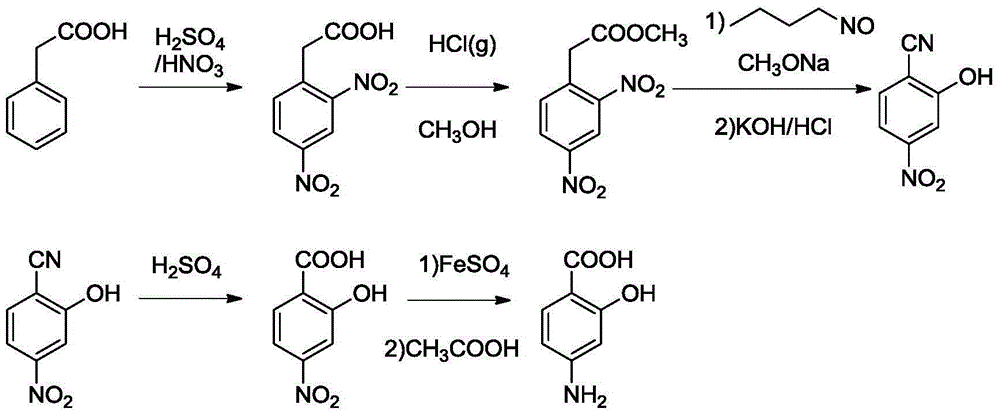
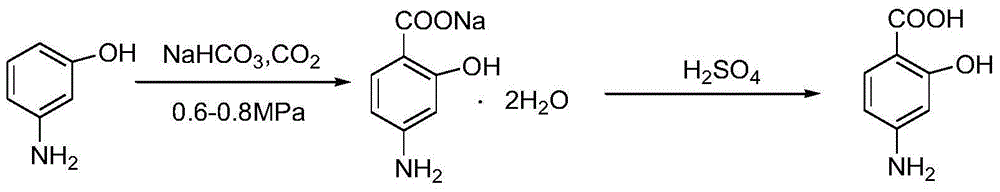
[0071] Add 500ml of ethyl acetate into a clean and dry reaction bottle, start stirring, protect the reaction system with nitrogen, then add 108g of p-aminosalicylic acid, then heat up to control the temperature of the reaction solution at 50±2°C, and stir to dissolve. Filter, lower the temperature to -10±2°C, stir and crystallize for 1.0 hour, filter, and dry the obtained material at 50±2°C to obtain 91.8g of p-aminosalicylic acid crystal form A with a purity of 99.91% and a yield of 85%. X-ray powder diffraction test pattern see attached figure 1 shown.
[0072] The X-ray powder diffraction characteristic absorption peak of p-aminosalicylic acid crystal form A obtained in Example 1 of Table 1
[0073] 2θ
Embodiment 2
[0075] Add 500ml of ethyl acetate into a clean and dry reaction bottle, start stirring, protect the reaction system with nitrogen, then add 108g of p-aminosalicylic acid, then heat up to control the temperature of the reaction solution at 50±2°C, and stir to dissolve. Filter, cool to 0±2°C, stir and crystallize for 1.0 hour, filter, and dry the obtained material at 50±2°C to obtain 89.6g of p-aminosalicylic acid crystal form A with a purity of 99.92% and a yield of 83%. The X-ray powder diffraction pattern is within the error range and figure 1 unanimous.
Embodiment 3
[0077] Add a mixed solvent of 350ml of tetrahydrofuran and 105ml of dichloromethane into a clean and dry reaction bottle, start stirring, protect the reaction system with nitrogen, then add 40g of p-aminosalicylic acid, then heat up to control the temperature of the reaction solution at 50±2°C, and stir dissolve clear. Filter, lower the temperature to -5±2°C, stir and crystallize for 1.0 hour, filter, and dry the obtained material at 50±2°C to obtain 29.6g of p-aminosalicylic acid crystal form A with a purity of 99.92% and a yield of 74%. The X-ray powder diffraction pattern is within the error range and figure 1 unanimous.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com