Novel fusion protein of glucagon-like peptide-1 (GLP-1) and human serum albumin as well as method for preparing fusion protein
A technology of human serum albumin and GLP-1, which is applied in the field of GLP-1 and HSA analogues, can solve the problems of enhanced side effects of patients, increased pain and side effects of patients, and high activity of drugs that do not consider the consistency of N-terminals. Convenient purification process and high expression yield
Inactive Publication Date: 2015-03-11
JIANGNAN UNIV +1
View PDF2 Cites 4 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Albiglutide is fused with fatty acid to become a long-acting drug. Because of its preparation method, the unit activity of the drug is lost by more than 100 times, while the half-life of the drug is only about 4-6 hours. Enhanced side effects
Exenatide is a single protein drug, and the biological preparation method makes the quality of the product well controlled, but the half-life of the drug is only 2 hours and the patient needs to inject it twice a day, which increases the pain and side effects of the patient
[0004] The recombinant protein of human serum albumin and GLP-1 analogue reported in the current study is expressed in yeast, and the problem of improving the high activity of the drug without considering the consistency of the N-terminal
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
Embodiment Construction
[0018] The specific implementation method is one of the preparation approaches of the present invention, and the present invention can also be implemented and applied through other approaches, and the present invention can be modified and improved without departing from the spirit of the present invention.
[0019] Specific implementation method 1: Cloning and preparation of stable strains of GLP-1 recombinant protein in CHO
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
 Login to View More
Login to View More Abstract
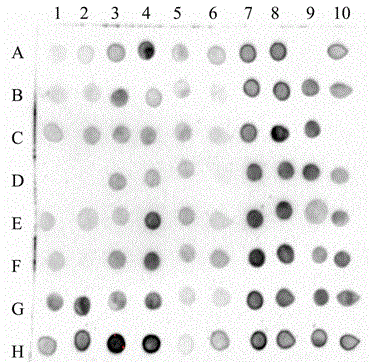
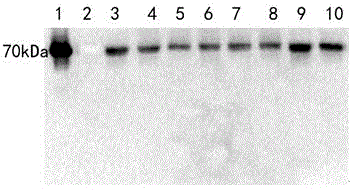
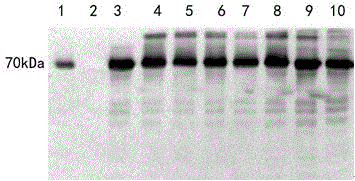
The invention provides a novel fusion protein of glucagon-like peptide-1 (GLP-1) analogue and human serum albumin as well as a method for preparing the fusion protein. The composition frame of the novel fusion protein is T-S-X-G-G-H, wherein the protein sequence represented by T is a biological affinity tag; the protein sequence represented by S is a protease enzyme cutting site; X represents an amino acid; G represents GLP-1 analogue protein; H represents human serum albumin. According to the design, a GLP-1 analogue sample, of which the N end is homogeneous, is obtained through biological preparation and enzyme digestion in sequence, so that the homogeneity and activity of a biological sample are improved. The invention further provides a Chinese hamster ovary cell (CHO) stable expressing cell strain capable of stably expressing the fusion protein. The novel fusion protein is higher in biological activity and better in vivo and vitro stability, can be used for treating 2-type diabetes mellitus and other diseases capable of being treated through fasting plasma glucose reduction, can be used for treating 1-type diabetes mellitus by inducing cells to differentiate into Beta pancreatic cells, and can be further used for treating other diseases capable of being treated through nervous system irritation causing satiety generation and peristole inhibition.
Description
technical field [0001] The invention utilizes genetic engineering means to design novel analogues of GLP-1 and HSA. Novel recombinant protein using the newly constructed high-efficiency expression plasmid pMH 3 , and expressed in Chinese hamster cells (CHO). A cell line with high activity and high expression (CHO / pMH 3 / TSXGGH). The yield of the obtained strain was as high as 9.45 μg*d -1 *10 6 cells. The fermentation expression yield is high, the purification process is convenient, and the product obtained by purification meets the requirements of raw materials. This drug is used in the treatment of diabetes and related diseases. Background technique [0002] Type 2 diabetes, also known as non-insulin-dependent diabetes, is due to low beta cell function, relative lack of insulin and insulin resistance. In recent years, the prevalence of type 2 diabetes has gradually increased. According to the World Health Organization's forecast, by 2030, there will be 300 million ...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More Patent Type & Authority Applications(China)
IPC IPC(8): C07K19/00C12N15/85C12P21/02A61P3/10
Inventor 金坚钱凯龚笑海马鑫朱瑞宇蔡燕飞陈蕴
Owner JIANGNAN UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com