Probes and kit for detecting human CYP2C9 (Cytochrome P450 2C9) and VKORC1 (Vitamin K epoxide reductase complex subunit 1) gene polymorphism
A technology for gene polymorphism and detection probes, which is applied in the determination/inspection of microorganisms, biochemical equipment and methods, DNA/RNA fragments, etc., can solve the problems of complicated operation, long detection process, high cost, and achieve linear detection Wide, high accuracy, the effect of eliminating pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
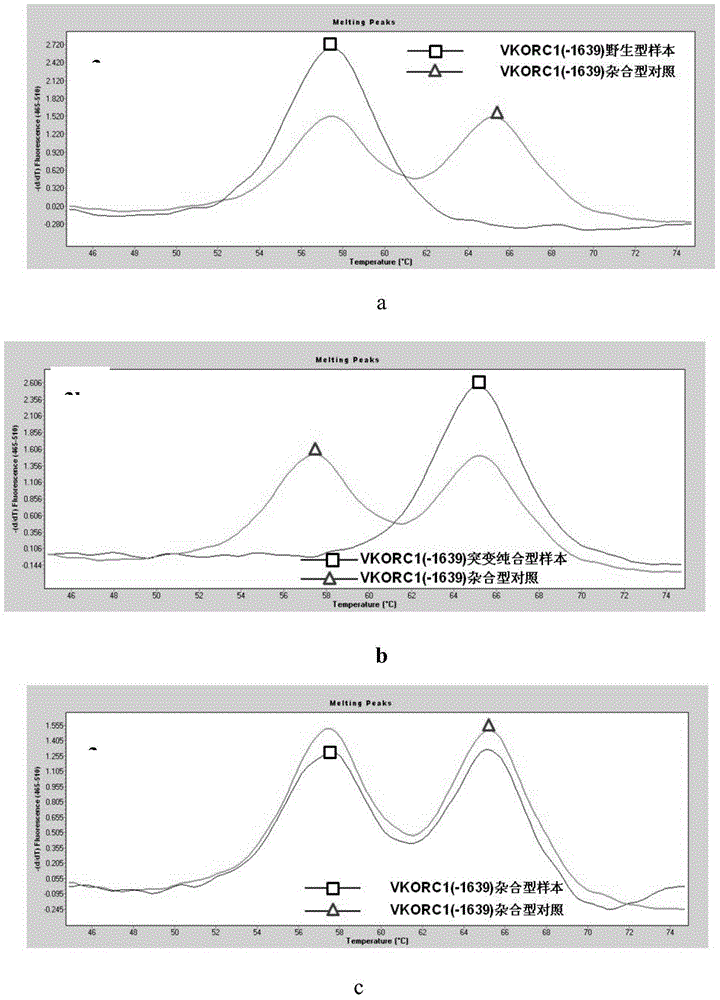
[0029] Example 1 CYP2C9 gene *2 (C430T) site, *3 (A1075C) site and VKORC1 gene (G-1639A) detection kit
[0030] This example designs specific primers and probes for CYP2C9 gene *2 (C430T) site, *3 (A1075C) site and VKORC1 gene (G-1639A), and reagents containing specific primers and specific probes box. The specific composition is as follows.
[0031] Table 1 Primer and probe sequences of human CYP2C9 and VKORC1 gene polymorphism detection kit
[0032]
[0033]
[0034] Using a kit including the above primers and fluorescent probes for simultaneous detection, the method is as follows:
[0035] 1. Sample processing and DNA extraction:
[0036]The test sample is EDTA anticoagulated whole blood sample, and the sample should be stored at room temperature for no more than 1 week, at 4°C for no more than 1 month, and at -20°C for no more than 7 weeks, and the blood sample should be frozen and thawed no more than 5 times. Use Guangzhou Haozhi DNA Extraction Kit (Cat. No.: DT...
Embodiment 2
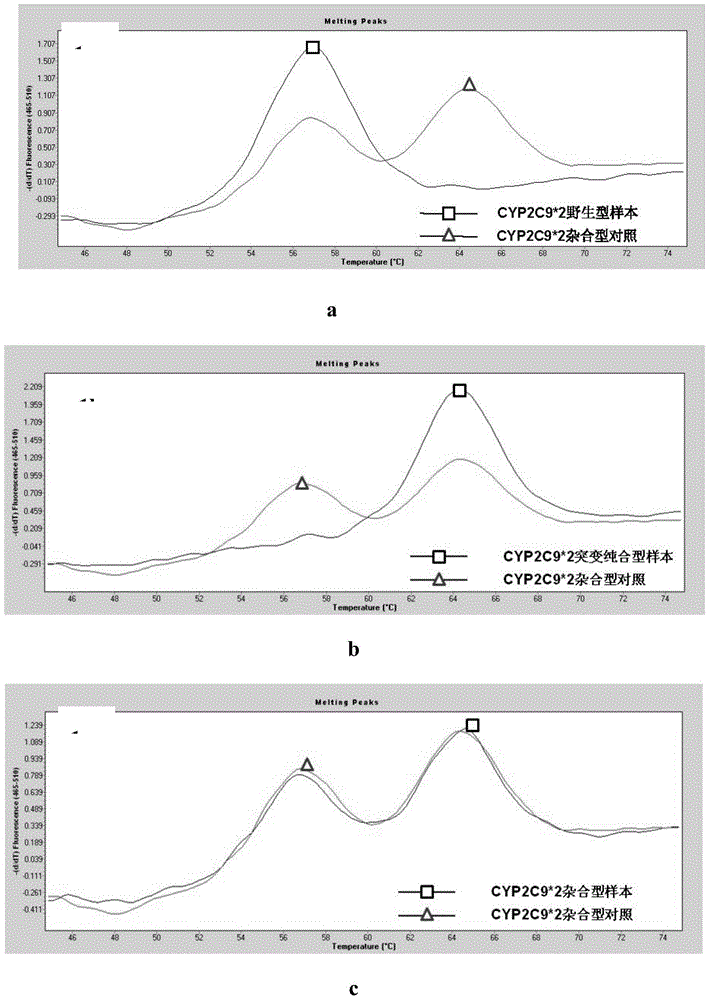
[0055] In this example, blood clinical samples (concentration range of 2ng / μL-60ng / μL) and CYP2C9*2 heterozygous plasmid (sequence derived from NCBI serial number: NG_008385.1) were used as standard products (plasmid diluted to 20fg / μL) As an example to illustrate the actual clinical sample detection results of the present invention.
[0056] The primers, probes and detection methods for the CYP2C9*2 locus are the same as those described in Example 1, and will not be repeated here.
[0057] The results showed that all types can be effectively interpreted, and the clinical samples were verified to be completely consistent in type by the gold standard Sanger method gene sequencing. The experimental results are attached figure 1 a, 1b, 1c.
Embodiment 3
[0059] In this example, blood clinical samples (concentration range of 2ng / μL-60ng / μL) and CYP2C9*3 heterozygous plasmid (sequence derived from NCBI serial number: NG_008385.1) were used as standard products (plasmid diluted to 20fg / μL) as Example illustrates the actual clinical sample detection results of the present invention.
[0060] The primers, probes and detection methods for the CYP2C9*3 locus are the same as those described in Example 1, and will not be repeated here.
[0061] The results showed that all types can be effectively interpreted, and the clinical samples were verified to be completely consistent in type by the gold standard Sanger method gene sequencing. The experimental results are attached figure 2 a, 2b, 2c.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com