A new midanate pharmaceutical composition and preparation method thereof
A technology of midanacin and composition, which is applied in the field of pharmaceutical composition for treating urinary incontinence, can solve problems such as low dissolution rate, poor stability, and poor content uniformity, and achieve fast dissolution rate, good stability, and reduced impurities the increased effect of
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
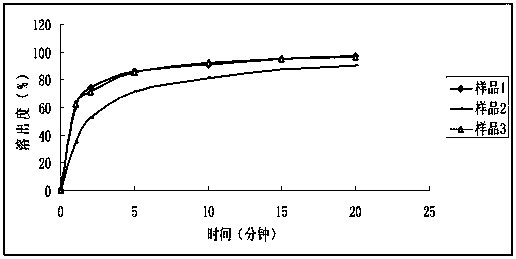
[0040] Content uniformity and dissolution rate comparison test
[0041] (1) Sample preparation
[0042] Preparation of Sample 1
[0043] Weigh 0.1g of midazine, add polyethylene glycol 200 4.0g, ultrasonically dissolve the midazine, add microcrystalline cellulose 302140g to fully mix, add 50g of pregelatinized starch as diluent, lubricant micropowder silica gel 1.0 g, after mixing evenly, press into tablets. Specification 0.1mg, tablet weight 200mg.
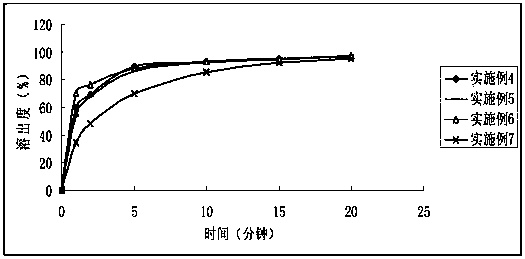
Embodiment 2
[0067] prescription
[0068] Accurately weigh 0.1 g of midanacin, add 2.0 g of polyethylene glycol 200, and sonicate for 10 minutes to dissolve the midanacin. Add microcrystalline cellulose to midanacin polyethylene glycol solution, mix at high speed for 2 minutes, add part of pregelatinized starch, mix at high speed for 1 minute, add binder (HPMC50 5% solution) at high speed After shearing and granulating, after obtaining suitable granules, take the material out of the equipment, dry it in an oven at 50°C for 3 hours, and sieve through a 20-mesh sieve for granulation. Add 0.2% magnesium stearate and mix and compress into tablets.
[0069] Table 4 Dissolution test results
[0070]
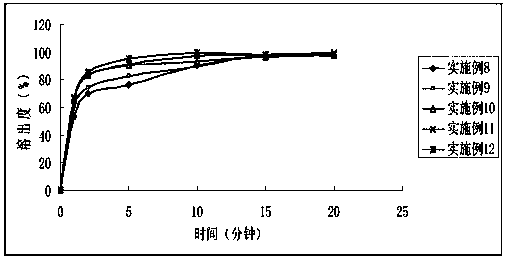
Embodiment 3
[0072] Midana new oral disintegration tablet
[0073]
[0074] Make a solution of the prescribed amount of midanacin solvent in polyethylene glycol, add microcrystalline cellulose to absorb the solution, add microscopic silica gel to absorb the excess solution, and obtain a fluid powder; add mannitol, sodium carboxymethylcellulose, Aspartame, the mixed powder is fully mixed with a high-speed shear mixing granulator, the mixed powder is added to the lubricant magnesium stearate and then compressed, and the pressure is adjusted to make the tablet hardness about 4kg.
PUM
| Property | Measurement | Unit |
|---|---|---|
| hardness | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com