Composition for treating degenerative disease
A degenerative disease and composition technology, applied in the field of composition for the treatment of degenerative diseases, can solve problems such as unsatisfactory therapeutic effects, delay the progress of degenerative diseases, make up for insufficient synthesis and secretion in the body, and inhibit the death of nerve cells Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
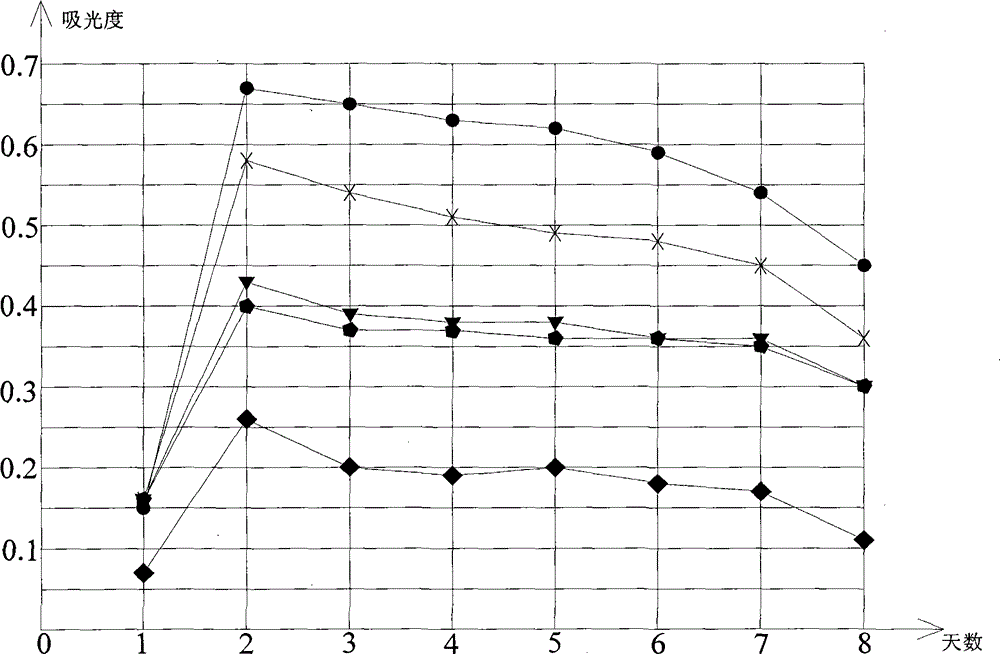
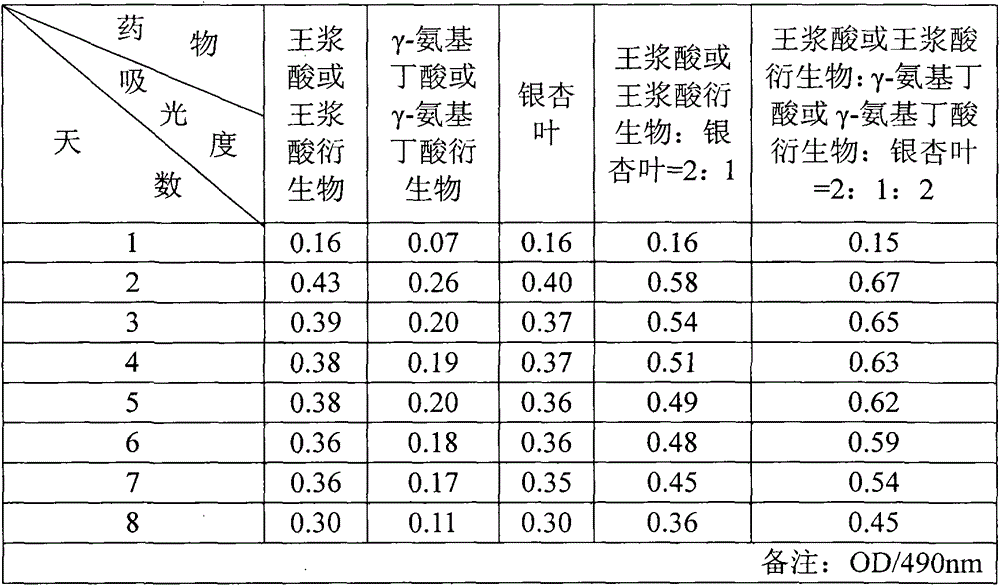
[0026] Embodiment 1, according to the ratio of royal jelly acid: gamma-aminobutyric acid: ginkgo leaf=2:1:2, royal jelly acid, gamma-aminobutyric acid, ginkgo leaf are weighed accurately, after fully mixing, pack into hard capsule, every Packed in 200mg capsules, each capsule contains royal jelly acid ≥ 75mg, γ-aminobutyric acid ≥ 38mg, ginkgo biloba flavonoids ≥ 17.5mg, ginkgo bilolides ≥ 4.0mg.
Embodiment 2
[0027] Embodiment 2, according to the ratio of royal jelly acid: gamma-aminobutyric acid: ginkgo leaf=1:1:3, royal jelly acid, gamma-aminobutyric acid, ginkgo leaf are weighed accurately, after fully mixing, pack into hard capsule, every Packed in 250mg capsules, each capsule contains royal jelly acid ≥ 45mg, γ-aminobutyric acid ≥ 45mg, ginkgo leaf flavonoids ≥ 35mg, ginkgo bilolides ≥ 8mg.
Embodiment 3
[0028] Embodiment 3, ferrous royal jelly, gamma-aminobutyric acid calcium, ginkgo leaf, starch are weighed according to the ratio of ferrous royal jelly: gamma-aminobutyric acid calcium: ginkgo leaf: starch=6:5:30:9 Accurate, fully mixed, made into tablets, each tablet 250mg, each tablet contains royal jelly acid ≥ 50mg, γ-aminobutyric acid ≥ 40mg, ginkgo leaf flavonoids ≥ 3mg, ginkgo biloba lactones ≥ 0.6mg.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com