Preparation and application of DMAP hydrochloride as catalyst of recoverable acylation reaction
A technology of hydrochloride and acylation is applied in the field of preparation and application of DMAP hydrochloride as a recyclable acylation catalyst, which can solve the problems of inapplicability of acylation reagents and damage to the structure.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Embodiment 1: the synthesis of DMAP hydrochloride:
[0019] Add 30 mL of toluene and 5.0 g (40.9 mmol) of DMAP into a 100 mL round-bottomed flask, and stir it electromagnetically to dissolve it. 2.92 g (80 mmol) of dry HCl gas was introduced into the system, and a white solid precipitated out of the system immediately. React at room temperature for 1 h, filter with suction, and dry in vacuo to obtain 6.28 g of white solid, yield 96.9%, melting point: 220—221°C. 1 H NMR (400MHz, CDC13) δ15.45(br, 1H), 8.14(t, J=6.8Hz, 2H), 6.78(d, J=6.8Hz, 2H), 3.27(s, 6H). 13 C NMR (100MHz, CDCl 3 )δ138.6, 106.8, 40.3.
Embodiment 2
[0020] Embodiment 2: the pivaloylation reaction of 1-cyano cyclohexanol:
[0021] Add 2.50 g (20 mmol) of 1-cyanocyclohexanol, 0.16 g (1 mmol) of DMAP hydrochloride and 2.64 g (22 mmol) of pivaloyl chloride into a 10 mL single-necked bottle. Control the reaction temperature to 110° C., react for 4 hours, and cool the reaction system to room temperature. Add 20 mL of n-hexane to the reaction system, filter and recover the catalyst, dry it in vacuum and put it into the next cycle. The filtrate was washed with 20mL water, 20mL saturated sodium bicarbonate, 15mL saturated brine, MgSO 4 Drying, precipitation under reduced pressure, and flash column chromatography yielded 3.97 g of a colorless oily product, with a yield of 95.0%. 1 H NMR (400MHz, CDCl 3 ( s, 9H).
Embodiment 3
[0022] Embodiment 3: the benzoylation reaction of o-nitrophenol:
[0023] Add 2.78g (20mmol) of o-nitrophenol, 20mL of toluene, 0.16g (1mmol) of DMAP hydrochloride and 4.52g (22mmol) of benzoic anhydride in a 50mL single-necked bottle. Control the reaction temperature to 60° C., react for 4 hours, and cool the reaction system to room temperature. Add 20 mL of n-hexane to the reaction system, filter and recover the catalyst, dry it in vacuum and put it into the next cycle. The filtrate was washed with 20mL water, 20mL saturated sodium bicarbonate, 15mL saturated brine, MgSO 4 Drying, desolvation under reduced pressure, and flash column chromatography yielded 4.67 g of the product as a pale yellow oil, with a yield of 96.1%. 1 H NMR (400MHz, CDCl 3 )δ8.25—8.18 (m, 2H), 8.16 (dd, J=8.0, 1.6Hz, 1H), 7.75—7.66 (m, 2H), 7.54 (t, J=7.6Hz, 2H), 7.48—7.43 (m, 1H), 7.43—7.37 (m, 1H).
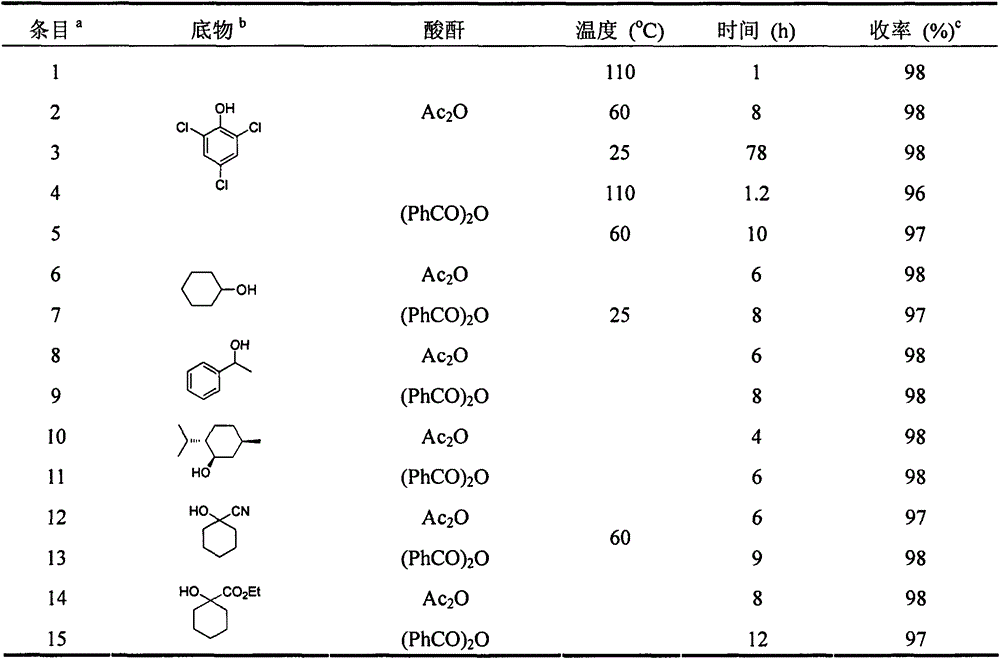
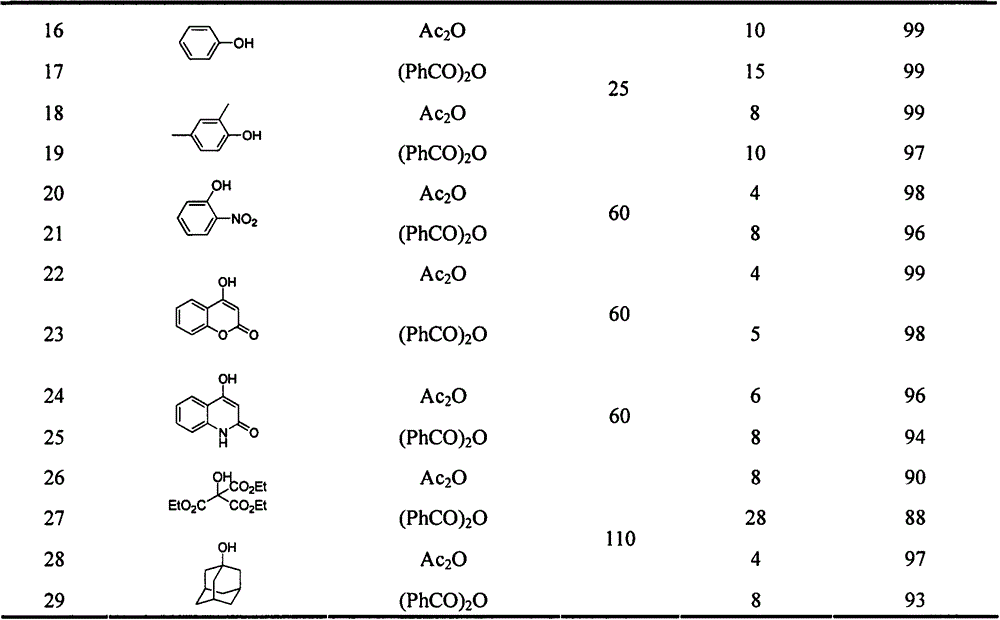
[0024] The following acylation products can be synthesized by the same method, see Table 1 and Ta...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com