Method for preparing Boc-L-tyrosine by using (Boc)2O
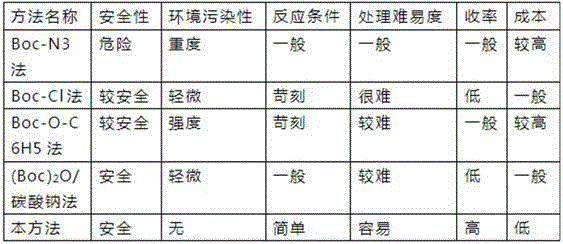
A boc-l-, tyrosine technology, applied in chemical instruments and methods, preparation of organic compounds, preparation of carbamate derivatives, etc., can solve the problems of low yield, difficult separation and purification, etc., and achieves simple and cost-effective methods. Inexpensive, high-yield effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] 1. Add 18.1g L-tyrosine and 100ml water into the reaction flask and stir. Add 5mol / L lye of 16g sodium hydroxide configuration to adjust to alkaline. Then add 8g of (Boc) 2 O reacted for 1 hour, then added 8g of (Boc) 2 O was reacted for 1 hour, and finally 9g of (Boc) was added 2 O was reacted for 3 hours.
[0034] 2. Use petroleum ether 10ml / time to extract impurities three times. After adjusting the pH to 3 with 3mol / L hydrochloric acid, extract the product three times with ethyl acetate 0.5L / time. The combined ester layers were washed with brine until neutral. Add 15 g of anhydrous sodium sulfate and dry for 6 hours.
[0035] 3. Filtrate, concentrate the filtrate to dryness under reduced pressure, add 50ml petroleum ether and stir to crystallize. The product is centrifuged and dried. 25.9 g of product was obtained. Yield 92.17%.
Embodiment 2
[0037] 1. Add 1kgL-tyrosine and 5.5L water into the reaction flask and stir. Add 10mol / L lye of 884g potassium hydroxide configuration to adjust to alkaline. Then add 442g of (Boc) 2 O reacted for 2 hours, then added 442g of (Boc) 2 O reacted for 2 hours, and finally added 497g of (Boc) 2 O was reacted for 4 hours.
[0038] 2. Use n-hexane 500ml / time to extract impurities four times. After adjusting the pH to 3 with 6mol / L hydrochloric acid, extract the product four times with tert-butyl acetate 1L / time. The combined ester layers were washed with brine until neutral. Add 300 g of anhydrous sodium sulfate and dry for 8 hours.
[0039] 3. Filtrate, concentrate the filtrate to dryness under reduced pressure, add 2L of n-hexane and stir to crystallize. The product is filtered off and dried. The product 1402g was obtained. Yield 90.29%.
Embodiment 3
[0041] 1. Add 10kgL-tyrosine and 55L water into the reaction flask and stir. Add 7mol / L lye of 8.9kg potassium hydroxide configuration to adjust to alkaline. Then add 4.4kg of (Boc) 2 O reacted for 1 hour, then added 4.4kg of (Boc) 2 O reacted for 1 hour, and finally added 5kg of (Boc) 2 O was reacted for 3 hours.
[0042] 2. Use n-pentane 5L / time to extract impurities five times. After adjusting pH=3 with 5mol / L hydrochloric acid, extract the product five times with 10L / time of dichloromethane. The combined ester layers were washed with brine until neutral. Add 3 kg of anhydrous sodium sulfate and dry for 15 hours.
[0043] 3. Filtrate, concentrate the filtrate to dryness under reduced pressure, add 20L n-pentane and stir to crystallize. The product is filtered off and dried. 14.1 kg of product were obtained. Yield 90.77%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com