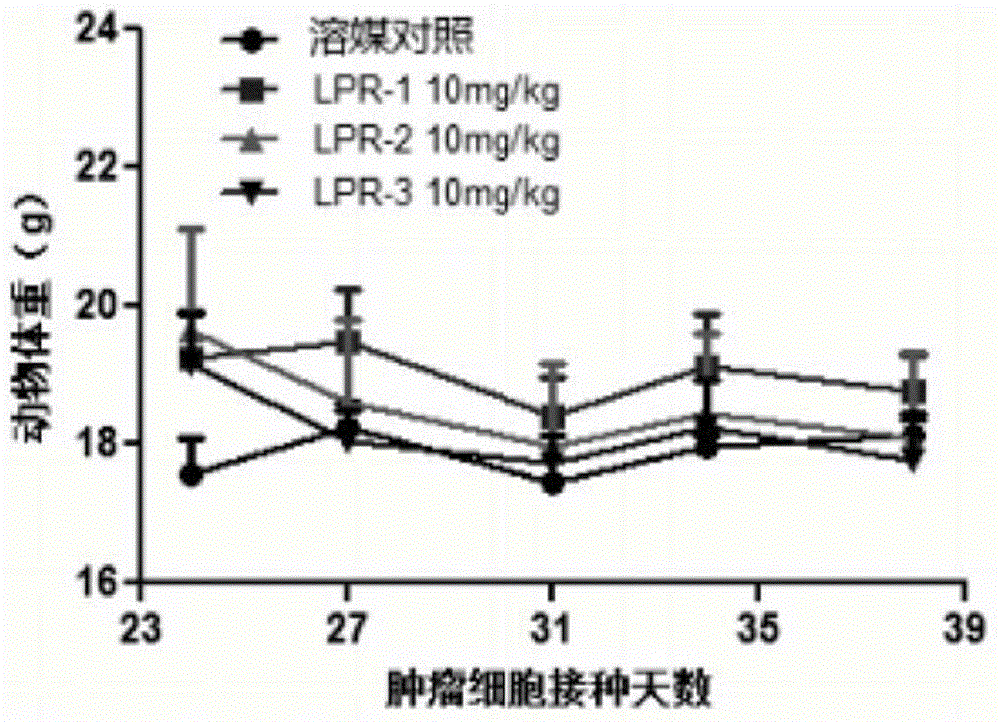
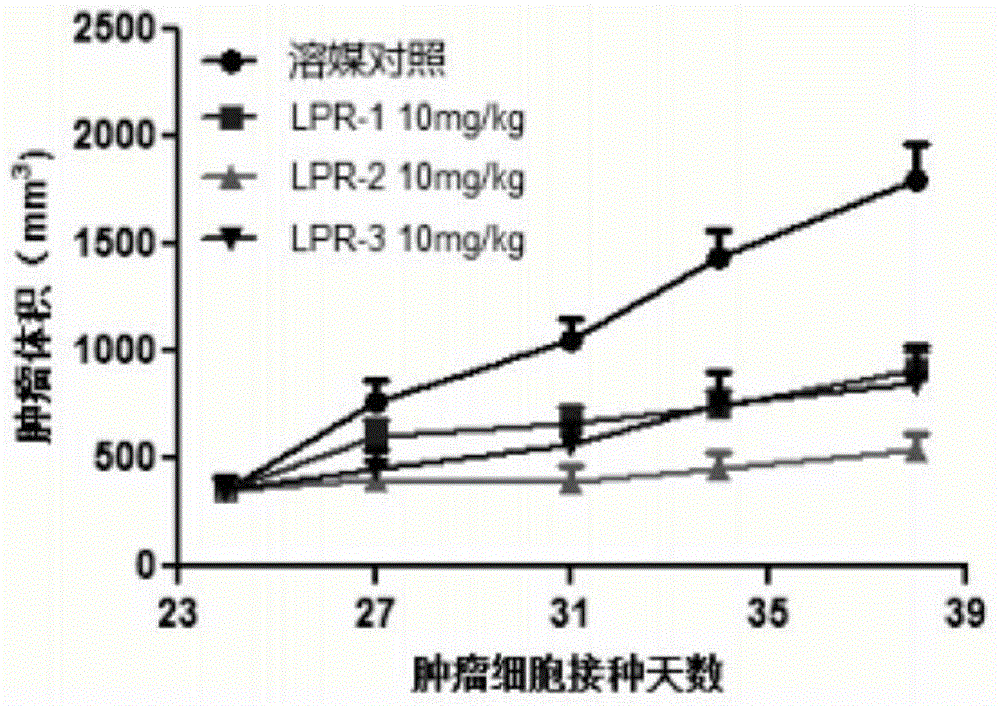
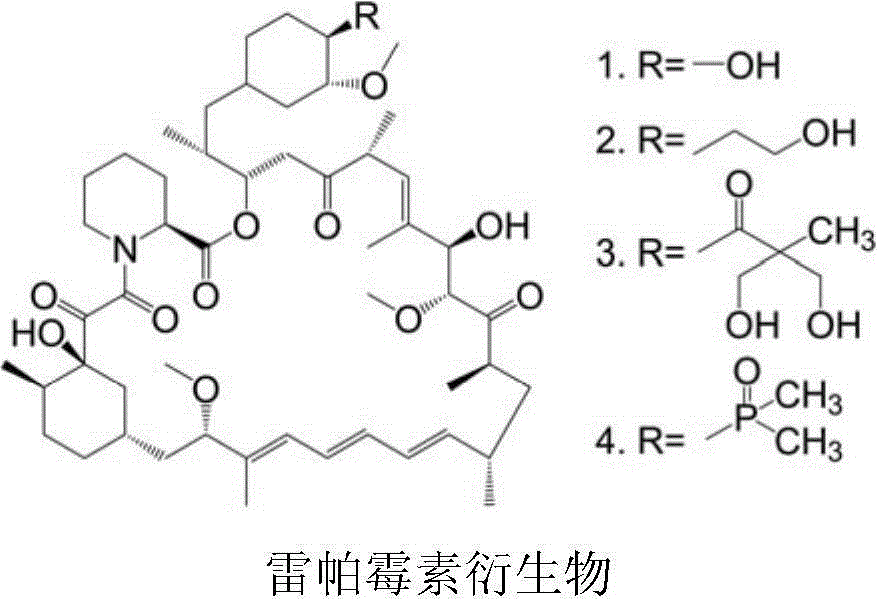
Polyethylene glycol-cactus oligopeptide bonded rapamycin derivative
一种化合物、多羧基的技术,应用在雷帕霉素衍生物领域,能够解决易反应不完全、反应产物复杂化、负载率降低等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0107] Example 1 Preparation of glycine rapamycin ester
[0108]
[0109] Add tert-butyl bromoacetate (5.82g, 30mmoL) to the reaction flask, dissolve it with acetone (80mL), then add a solution of sodium azide (4.55g, 70mmoL) dissolved in water (40mL), and heat to reflux overnight. The reaction solution was evaporated to remove acetone, and the residue was extracted with ether. The extract was washed with saturated brine, dried, and concentrated under reduced pressure to obtain an oily liquid. This liquid was dissolved in methanol (90 mL), and 1N sodium hydroxide solution (90 mL) was added, stirred, and heated to reflux for 3 h. After cooling, the methanol was evaporated under reduced pressure, the remaining night was cooled in an ice bath, and 6N hydrochloric acid was added to adjust the pH to 2, and then extracted with ether. The extract was washed with water, dried, and concentrated to obtain azidoacetic acid, MS m / z: 124[ M+Na] + .
[0110] Add azidoacetic acid (253mg, 2.5mmo...
Embodiment 2
[0112] Example 2 Preparation of monomethoxy polyethylene glycol (number average molecular weight 20000)-rapamycin conjugate (LPR-1)
[0113]
[0114] Mix monooxypolyethylene glycol acetic acid (20K, 1g, 0.05mmoL), glycine rapamycin ester (97mg, 0.1mmoL) prepared in Example 1, and 1-hydroxybenzotriazole (HOBt, 6.8mg, Add 0.05mmoL) and DMAP (12.2mg, 0.1mmoL) to the reaction flask, dissolve with dichloromethane, cool in an ice bath, and then drop in a solution of DCC (15.5mg, 0.075mmoL) dissolved in dichloromethane. It was naturally raised to room temperature and reacted overnight. The next day, the reaction solution was concentrated, and the residue was crystallized with isopropanol to obtain monomethoxy polyethylene glycol (20K)-rapamycin conjugate (LPR-1) 0.82g (n Approximately 450).
[0115] 1 H-NMR(300MHz, CDCl 3 ): 0.90 (Me, 3H, 43), 0.92 (Me, 3H, 49), 0.94 (Me, 3H, 46), 0.96 (Me, 3H, 48), 0.97 (Me, 3H, 45), 1.10 (CH 2 , 2H, 24), 1.11(CH 2 , 2H, 36), 1.20(CH 2 , 2H, 42), 1.33(C...
Embodiment 3
[0116] Example 3 Preparation of monomethoxy polyethylene glycol (number average molecular weight 20000)-glutamic acid dipeptide-rapamycin conjugate (LPR-2)
[0117]
[0118] The monomethoxypolyethylene glycol-glutamic acid dipeptide (20K, 0.5g, 0.025mmol), 48.6mg (0.05mmol) of glycine rapamycin ester prepared in Example 1, HOBt (3.4mg, 0.025mmoL) ) And DMAP 6.1mg (0.05mmoL) were added to the reaction flask, dissolved in dichloromethane, cooled in an ice bath, and then dripped with a solution of 15.5mg (0.075mmol) of DCC dissolved in dichloromethane, and naturally warmed to room temperature after dripping , React overnight. The next day, the reaction solution was concentrated, and the residue was crystallized with isopropanol to obtain monomethoxy polyethylene glycol (20K)-glutamic acid dipeptide-rapamycin conjugate (LPR-2) 0.41g (n about For 450).
[0119] 1 H-NMR(300MHz, CDCl 3 ): 0.90 (Me, 9H, 43), 0.92 (Me, 9H, 49), 0.94 (Me, 9H, 46), 0.96 (Me, 9H, 48), 0.97 (Me, 9H, 45), 1.10...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com