Stabilizer for heavy metal compound contaminated soil and preparation method of stabilizer for heavy metal compound contaminated soil
A stabilizer and heavy metal technology, applied in the field of soil remediation, can solve the problems of inconspicuous stabilization effect, promotion of arsenic release, poor buffer capacity, etc., and achieve the effects of overcoming poor stabilization effect, realizing resource utilization, and reducing leaching concentration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] 1. Selection of contaminated soil
[0048] The soil samples were collected from a polluted site in Gansu. The basic properties of the soil are shown in Table 1. The leaching concentration of Pb in the soil sample was 40.37mg / L, and the leaching concentration of Zn was 229.39mg. / L, the leaching concentration of Cd is 8.56mg / L, and the leaching concentration of As is 0.03mg / L; the total effective state content of the heavy metals lead, zinc, and cadmium to be stabilized and the effective state content of arsenic are calculated.
[0049] Table 1
[0050]
[0051] 2. Selection of raw materials:
[0052] (1) Sodium sulfide: commonly known as soda sulfide, the chemical formula is Na 2 S·9H 2 O, content ≥ 98%, produced by Shanghai Tongya Chemical Technology Development Co., Ltd.;
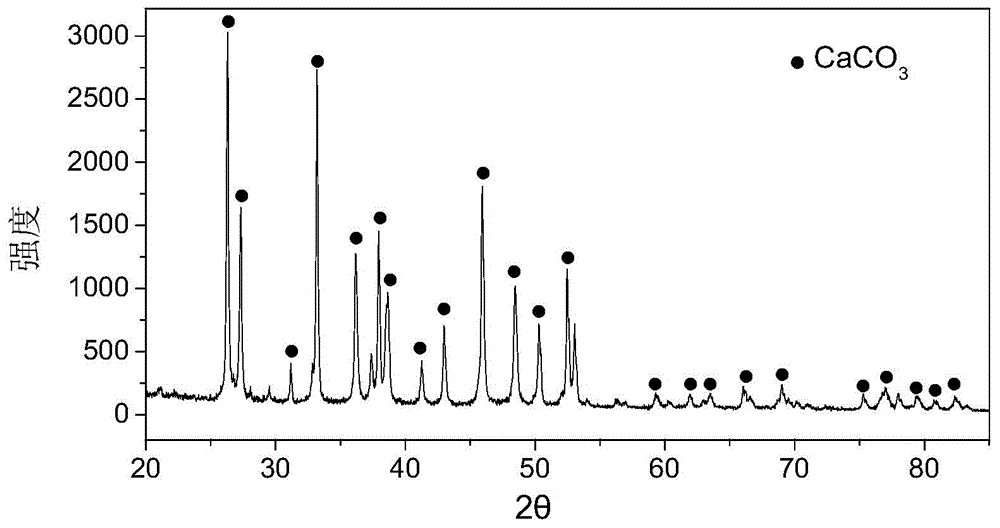
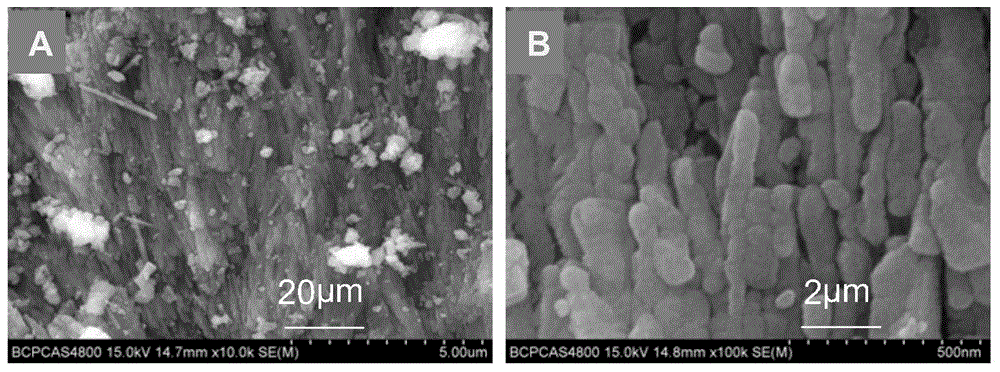
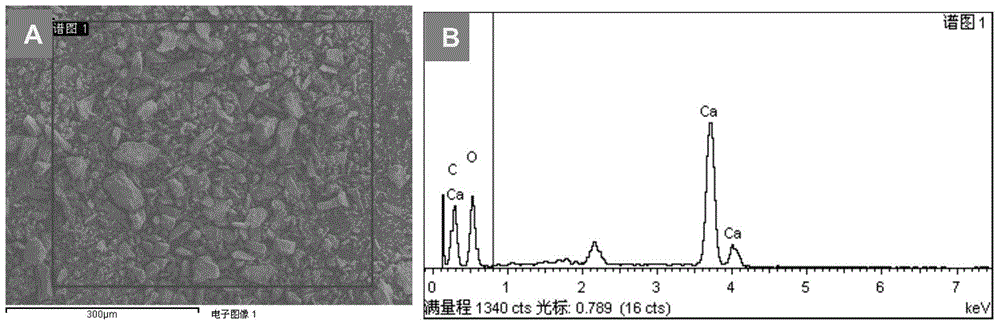
[0053] (2) Shell powder: wash the shells with water, dry them, and grind them through a 400-mesh (38 μm) sieve. Mineral composition of shell flour see figure 1 ; Microscopic morphology and s...
Embodiment 2
[0063] The selection of polluted soil and raw materials is the same as in Example 1, and the preparation of the stabilizer is different from Example 1.
[0064] Sodium sulfide is added at a molar ratio of 3 times the total amount of heavy metal lead, zinc, and cadmium; shell powder is added at a molar ratio of 2 times the total amount of heavy metal lead, zinc, and cadmium; Add in a molar ratio of 4 times the effective state of arsenic.
[0065] The preparation method and use method of the stabilizer are the same as in Example 1, and the addition amount of the stabilizer is 5% of the soil weight. Seal and place the uniformly mixed sample in a standard curing box under the conditions of temperature 20±2°C and relative humidity 95%. After curing for 3 days, do toxicity leaching test. The test method is carried out according to the leaching method of the US Environmental Protection Agency TCLP, and the leaching results are as follows: Figure 5 shown.
[0066] From Figure 5...
Embodiment 3
[0068] The selection of polluted soil and raw materials is the same as in Example 1, and the preparation of the stabilizer is different from Examples 1 and 2.
[0069] Add sodium sulfide at a molar ratio of 6 times the total amount of available heavy metal lead, zinc, and cadmium; add shell powder at a molar ratio that is 4 times the total amount of available heavy metal lead, zinc, and cadmium; Add in a molar ratio of 8 times the effective state of iron to arsenic.
[0070] The preparation method and use method of the stabilizer are the same as in Example 1, and the addition amount of the stabilizer is 10% of the soil weight. Seal and place the uniformly mixed sample in a standard curing box under the conditions of temperature 20±2°C and relative humidity 95%. After curing for 3 days, do toxicity leaching test. The test method is carried out according to the leaching method of the US Environmental Protection Agency TCLP, and the leaching results are as follows: Figure 6 s...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com