Mesoporous chromium-oxide-based catalyst for dehydrohalogenation reaction
A chromium-based catalyst, dehydrohalogenation technology, applied in the direction of dehydrohalogenation preparation, physical/chemical process catalyst, metal/metal oxide/metal hydroxide catalyst, etc., can solve poor thermal stability, component loss, specific Small surface area and other issues, to achieve the effect of rich mesoporous structure, high stability and large specific surface area
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060] Example 1: Preparation of mesoporous chromium oxide by template method
[0061] Dissolve a mixture of 10.0g trivalent chromium soluble salt, 30.0g urea, 15.0g templating agent and 20.0g ionic strength regulator in water, and reflux at 100°C for 9 hours with stirring to allow it to fully precipitate, and then suction-filter to obtain a precipitated solid , washed with deionized water until neutral, and then dried overnight at 100 °C to obtain a mesoporous chromium oxide precursor. Then the body was roasted in a muffle furnace for 8 hours to obtain mesoporous chromium oxide.
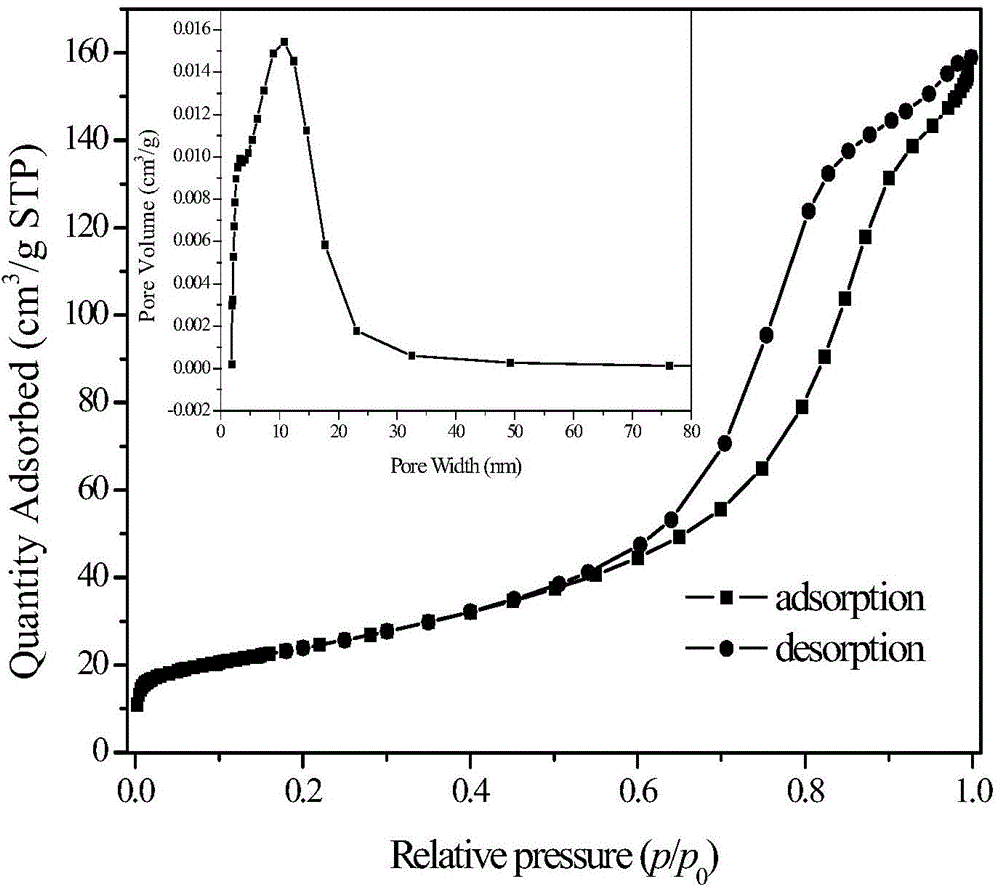
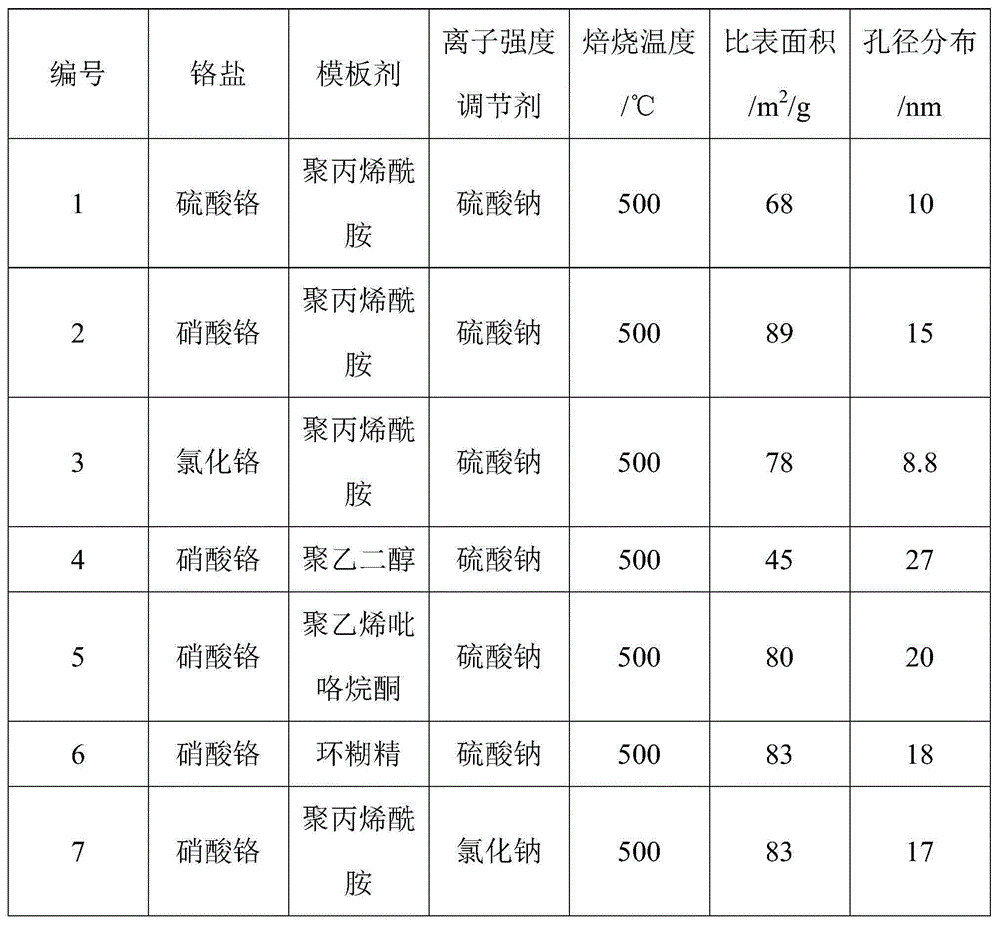
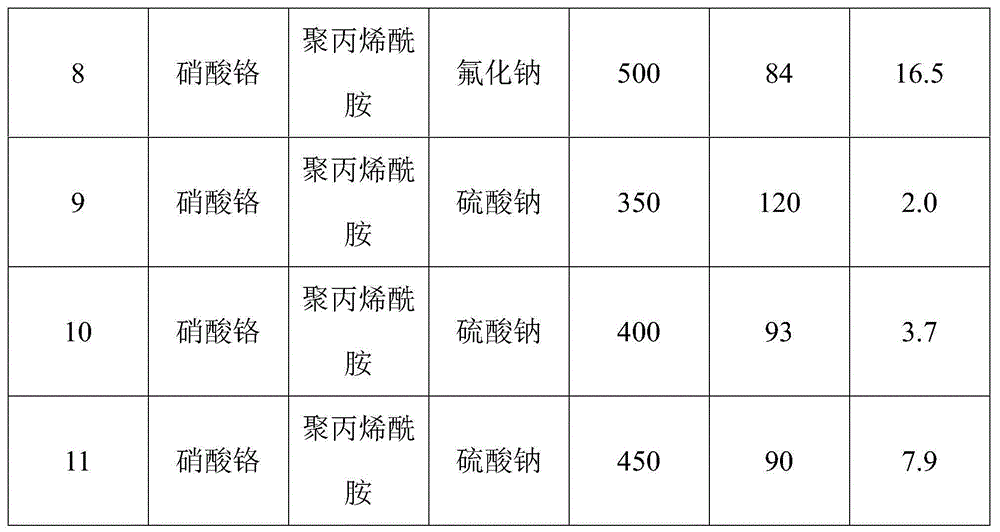
[0062] Catalyst N 2 Adsorption and desorption performance test: the chromium oxides prepared under different chromium soluble salts, template agents, ionic strength regulators and roasting temperatures and their specific surface areas and pore size distributions are shown in Table 1, wherein the isothermal The adsorption-desorption curve is as figure 1 shown.
[0063] Catalyst physicochemical pr...
Embodiment 2
[0067] Dissolve 8.0g of fluorides corresponding to alkali metals Na, K, Rb, and Cs in 100ml of distilled water to make a solution, and then add 2 / g of mesoporous chromium oxide, impregnated for 6 hours, dried at 80°C for more than 12 hours, and then roasted the precursor in a muffle furnace at 400°C for 6 hours to obtain a chromium oxide-based dehydrohalogenation catalyst.
[0068] A series of alkali metal-doped chromium oxide catalysts prepared as dehydrochlorination catalysts were filled with 60ml of catalysts in a fixed-bed tubular reactor with a diameter of Φ38mm. At a reaction temperature of 400°C, 2-chloro- 1,1,1,2-Tetrafluoropropane passed through the catalyst bed with a residence time of 24s. The product is washed with water and alkali to remove HCl and HF, and then analyzed by gas chromatography. The analysis method is the area normalization method. After 48 hours of reaction, the results of dehydrochlorination are shown in Table 2.
[0069] The reaction result of ...
Embodiment 3
[0073] Dissolve 10.0g of the corresponding nitrates of alkaline earth metals Mg, Ca, and Ba in 100ml of distilled water to make a solution, and then add 2 / g of mesoporous chromium oxide, impregnated for 6 hours, dried at 100°C for more than 12 hours, and then roasted the precursor in a muffle furnace at 500°C for 6 hours to obtain a chromium oxide-based dehydrohalogenation catalyst.
[0074] A series of alkaline earth metal-doped chromium oxide catalysts were prepared as dehydrochlorination catalysts, and 60ml of catalysts were loaded into a fixed-bed tubular reactor with a diameter of Φ38mm. At a reaction temperature of 400°C, 2,3- Dichloro-1,1,1-trifluoropropane passed through the catalyst bed with a residence time of 24s. The product is washed with water and alkali to remove HCl and HF, and then analyzed by gas chromatography. The analysis method is the area normalization method. After reacting for 48 hours, the results of dehydrochlorination are shown in Table 3.
[007...
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com