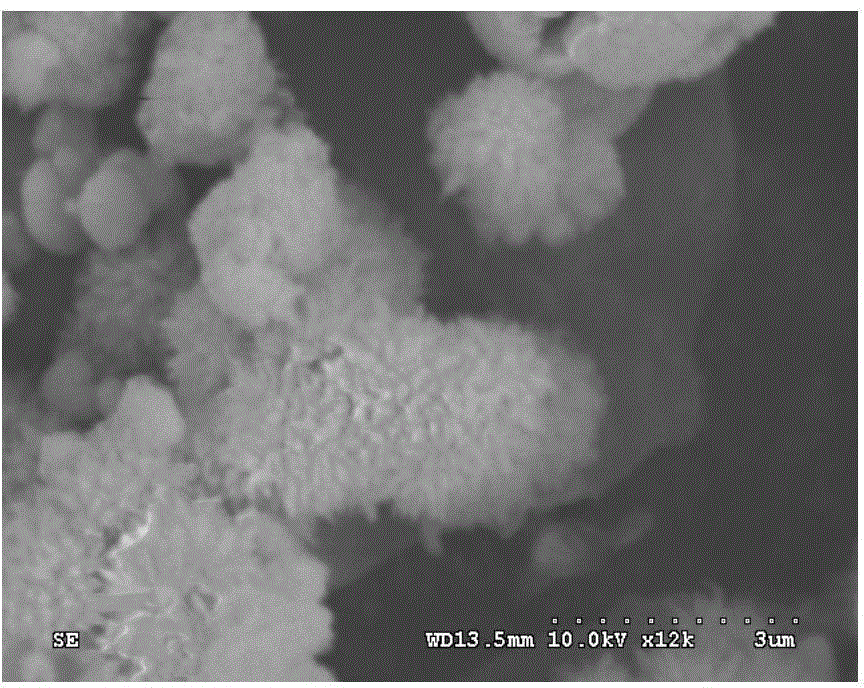
Preparation method for denitration catalyst
The technology of denitration catalyst and salt compound is applied in the field of preparation of denitration catalyst, which can solve the problems of small specific surface area of denitration catalyst, few internal pores of denitration catalyst, low utilization rate of effective active sites, etc. The effect of improving utilization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0027] The invention provides a method for preparing a denitration catalyst, including:
[0028] Reacting a manganese salt, a salt compound, and a thiosulfate salt in a solvent to obtain a reaction product, the salt compound including permanganate, iron salt or cerium salt;
[0029] The reaction product is calcined to obtain a denitration catalyst.
[0030] The present invention reacts manganese salt, salt compound and thiosulfate in a solvent to obtain a reaction product. In the embodiment of the present invention, the molar ratio of the manganese salt, the salt compound and the thiosulfate salt may be (1.5-10):1:(2.5-11); in other embodiments, the manganese salt The molar ratio of the salt compound and the thiosulfate salt may be (2-8):1:(3-8); in other embodiments, the molar ratio of the manganese salt, the salt compound and the thiosulfate salt The ratio can be (3~5):1:(4~6). In the present invention, there is no particular limitation on the amount of the solvent, as long as t...
Embodiment 1
[0052] 20.00g of manganese acetate and 3.05g of potassium permanganate were mixed with water to form a mixed solution, the molar concentration of manganese acetate in the mixed solution was 0.1 mol / L; while stirring, 67.4 was added dropwise to the above mixed solution mL of ammonium thiosulfate aqueous solution with a molar concentration of 2.0 mol / L was subjected to 2h aging treatment; the obtained aging treatment product was transferred to a polytetrafluoroethylene-lined reactor, and 0.5 was carried out at 150°C under static conditions. Hour response
[0053] The obtained reaction product was naturally cooled to room temperature, and the reaction product cooled to room temperature was subjected to suction filtration. The product after suction filtration was repeatedly washed with deionized water and absolute ethanol to neutrality, and the washed product was kept at 110°C. After drying for 4 hours, the dried product is calcined at 500° C. for 3 hours to obtain a denitration cata...
Embodiment 2
[0057] 18.00g of manganese sulfate and 5.52g of hydrated ferrous sulfate with water were prepared into a mixed solution, the molar concentration of manganese sulfate in the mixed solution was 0.15 mol / L; under stirring, 46.4 was added dropwise to the above mixed solution mL of ammonium thiosulfate solution with a molar concentration of 3.0 mol / L was subjected to 6 hours of aging treatment; the obtained aging treatment product was transferred to a polytetrafluoro-lined reactor, at 180 ℃, standing conditions for 2 Hour response
[0058] The obtained reaction product was naturally cooled to room temperature, and the reaction product cooled to room temperature was subjected to suction filtration. The product after suction filtration was repeatedly washed with deionized water and absolute ethanol to neutrality, and the washed product was kept at 110°C. After drying for 12 hours, the dried product is calcined at 350° C. for 4 hours to obtain a denitration catalyst.
[0059] The denitrat...
PUM
| Property | Measurement | Unit |
|---|---|---|
| denitrification rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com