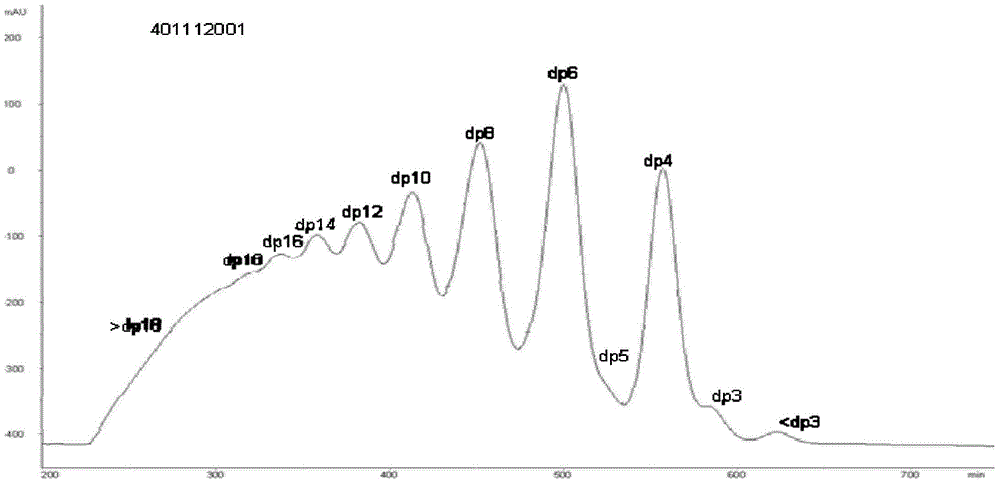
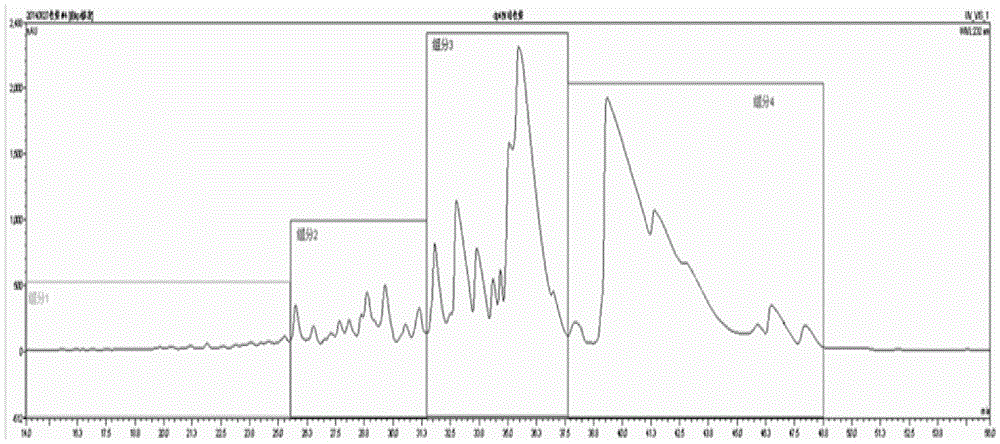
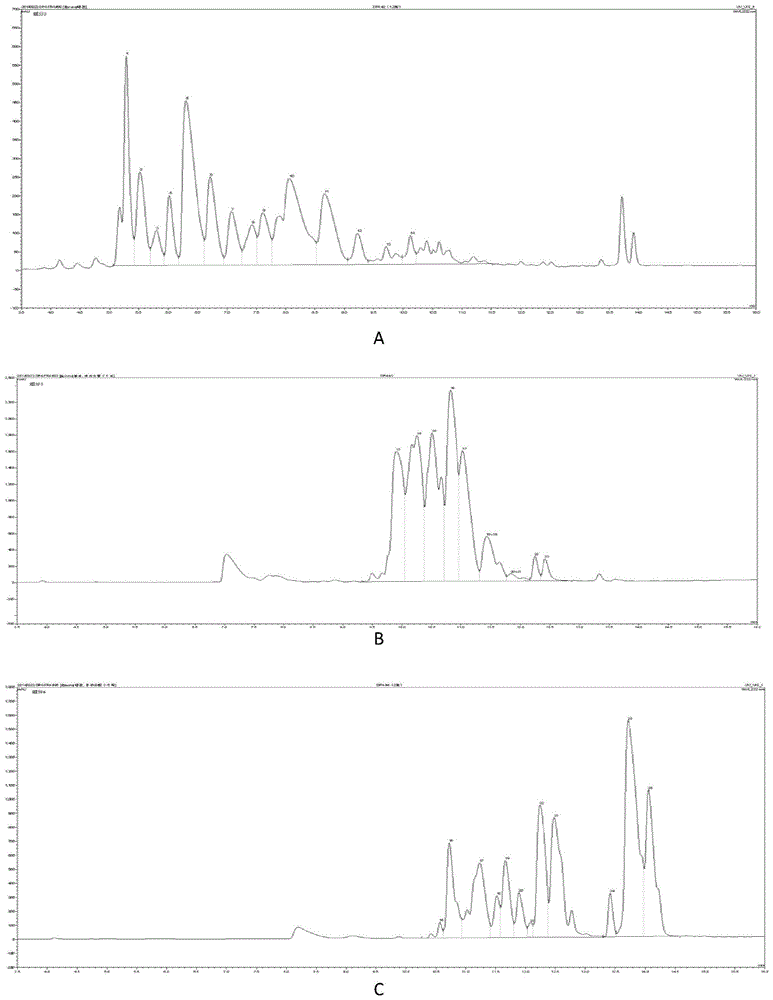
A method for collecting enoxaparin oligosaccharides by rp-ip-hplc method
A technology of enoxaparin oligosaccharide and enoxaparin sodium, applied in the field of medicine, can solve the problems of oligosaccharide sequence destruction, extensiveness, low purity, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0096] Preparation of solutions in each step of enoxaparin sodium oligosaccharide collection
[0097] Preparation of enoxaparin sodium sample solution: Weigh 1000 mg of enoxaparin sodium sample into a clean test tube, add 15 ml of ultrapure water to dissolve, and mix well.
[0098] Preparation of 10 mg / ml total tetrasaccharide component solution: Weigh 15 mg of the collected total tetrasaccharide component sample into a clean test tube, add 1.5 ml of ultrapure water to dissolve, and mix well.
[0099] Preparation of 1 mg / ml pre-separated tetrasaccharide component solution: Weigh 3 mg of pre-separated tetrasaccharide sub-component sample into a clean test tube, dissolve in 3 ml of ultrapure water, and mix well.
[0100] Preparation of 1 mg / ml tetrasaccharide three-seed component solution: Take 1 mg of the three-step segregation sub-component samples of tetrasaccharide respectively in a clean test tube, add 1 ml, and mix well.
[0101] Solution A: Weigh 1.03g of dibutylamine an...
Embodiment 2
[0130] The collection method of the present invention is compared with the existing enzymatic degradation-HPLC collection method and ultrafiltration collection method, respectively from the aspects of collection range, time, oligosaccharide yield, purity and collection cost, etc. The comparison is shown in Table 8 below:
[0131] Table 8
[0132]
[0133]
[0134]As can be seen from the comparative results in Table 8, by comprehensively considering aspects such as collection range, time, oligosaccharide yield, purity and collection cost, the collection method of the present invention is all compared with the existing enzymatic degradation-HPLC collection method and ultrafiltration The collection method is more economical and effective.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com