Preparation method for orotic acid
A technology of orotic acid and acetic acid, applied in the direction of organic chemistry, can solve the problems of high process cost and limited application of orotic acid
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
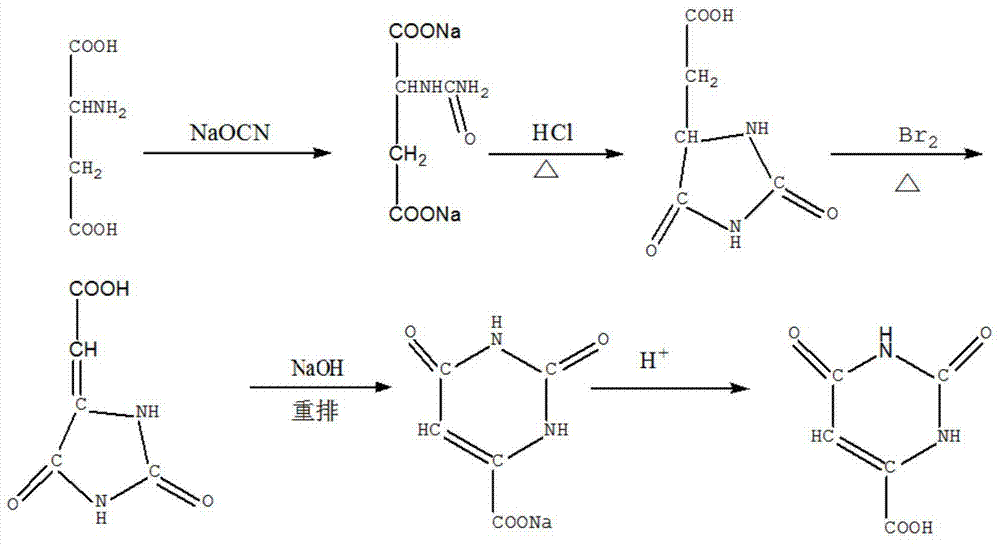
[0035] The invention provides a kind of preparation method of orotic acid, the preparation method of this orotic acid comprises the following steps:
[0036] (1) Synthesis of carbamoylaspartic acid: add 13.3 g of aspartic acid in the reaction vessel, then add 7 g of sodium cyanate, then add 39.9 ml of water, react at 60 ° C for 3 hours, add mass after natural cooling The concentration is 38% concentrated hydrochloric acid to pH 2.2, carbamoylaspartic acid is precipitated, the filter residue is obtained by filtration, and the filter residue is dried at 80-100°C to obtain 11 g of carbamoylaspartic acid, with a yield of 70% ;
[0037] (2) Synthesis of hydantoin acetic acid: in a reaction vessel, add 11 g of carbamoylaspartic acid, add 32 milliliters of 20 wt % hydrochloric acid, heat and evaporate to dryness, recrystallize the residue in water, and filter to obtain a filter residue , dried to obtain hydantoin acetic acid 10g, yield 81%;
[0038] (3) Synthesis of carboxymethylhy...
Embodiment 2
[0041] The invention provides a kind of preparation method of orotic acid, and its concrete steps are as follows:
[0042](1) Synthesis of carbamoylaspartic acid: add 26.6 g of aspartic acid in the reaction vessel, then add 14 g of sodium cyanate, then add 79.8 ml of water, react at 70 ° C for 4 hours, add mass after natural cooling The fraction is 38% concentrated hydrochloric acid to pH 2.3, carbamoylaspartic acid is precipitated, the filter residue is obtained by filtration, and the filter residue is dried at 80-100° C. to obtain 20 g of the product with a yield of 69%.
[0043] (2) Synthesis of hydantoin acetic acid: in a reaction vessel, add 20 g of carbamoylaspartic acid, add 60 milliliters of 20 wt % hydrochloric acid, heat and evaporate to dryness, recrystallize the residue in water, and filter to obtain a filter residue , drying the filter residue to obtain 18 g of hydantoin acetic acid with a yield of 79%.
[0044] (3) Synthesis of carboxymethylhydantoin: In a react...
Embodiment 3
[0047] The preparation method of orotic acid, its concrete steps are as follows:
[0048] (1) Synthesis of carbamoylaspartic acid: Add 39.9 g of aspartic acid in the reaction vessel, then add 21 g of sodium cyanate, then add 119.7 ml of water, react at 75 ° C for 4.5 hours, add mass after natural cooling The fraction is 38% concentrated hydrochloric acid until the pH is 2.3, and carbamoylaspartic acid is precipitated, filtered, and dried to obtain 28.5 g of the product, with a yield of 65%.
[0049] (2) Synthesis of hydantoin acetic acid: in a reaction vessel, add 28.5 g of carbamoylaspartic acid, add 85 milliliters of 20 wt% hydrochloric acid, heat and evaporate to dryness, recrystallize the residue in water, and filter to obtain The filter residue was dried to obtain 22.8 g of hydantoin acetic acid with a yield of 76%.
[0050] (3) Synthesis of carboxymethylhydantoin: In a reaction vessel, add 22.8 g of hydantoin acetic acid, then add 11.4 mL of liquid bromine, then add 45....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com