Synthetic method of menatetrenone
A technology of menadione and a synthesis method, applied in the field of medicine, can solve problems such as vitamin K deficiency, and achieve the effects of simple preparation method, low cost and optimized preparation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
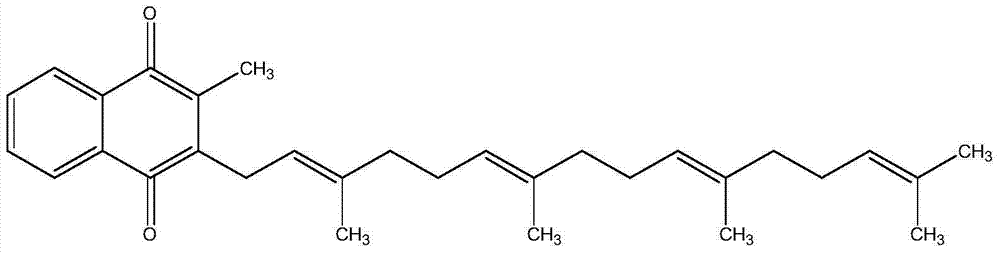
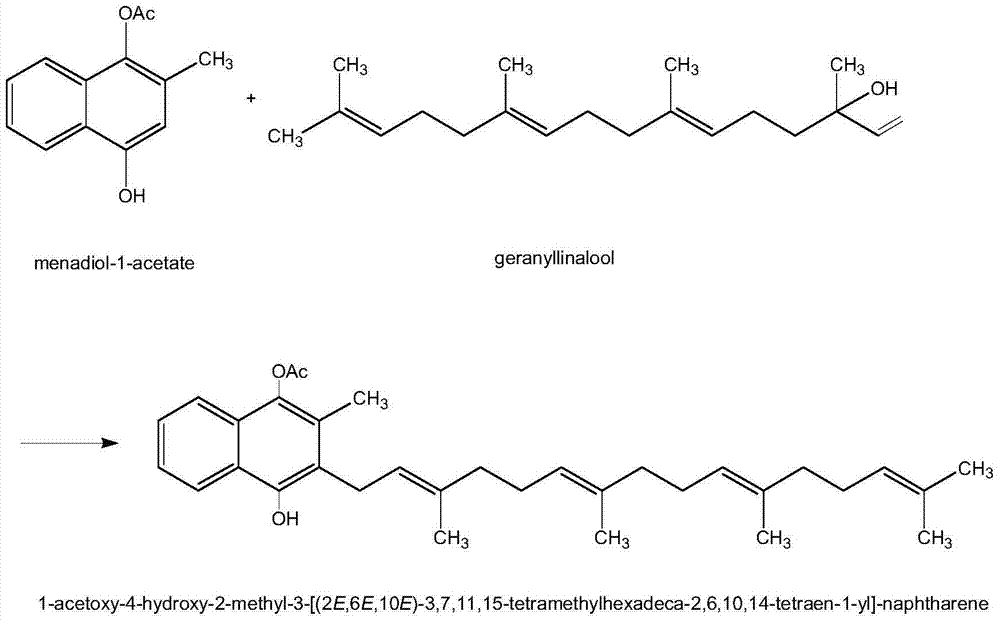
[0019] Step 1: Condensation Reaction
[0020] In a four-necked flask, add 10.0 g of menadione monoacetate, 35 ml of butyl ether, and 2 g of boron trifluoride·diethyl ether, heat to reflux, and start to add 6.5 g of geranyl kirinanol in stages. After 2 hours of reaction, the progress of the test reaction was followed until the reaction of geranyl kilinanol was completed. Washed with water, 5% sodium bicarbonate and 5% sodium chloride in turn, and washed with water until neutral, and discarded the aqueous layer.
[0021] The upper organic layer was concentrated, and the organic solvent was recovered until no solvent remained.
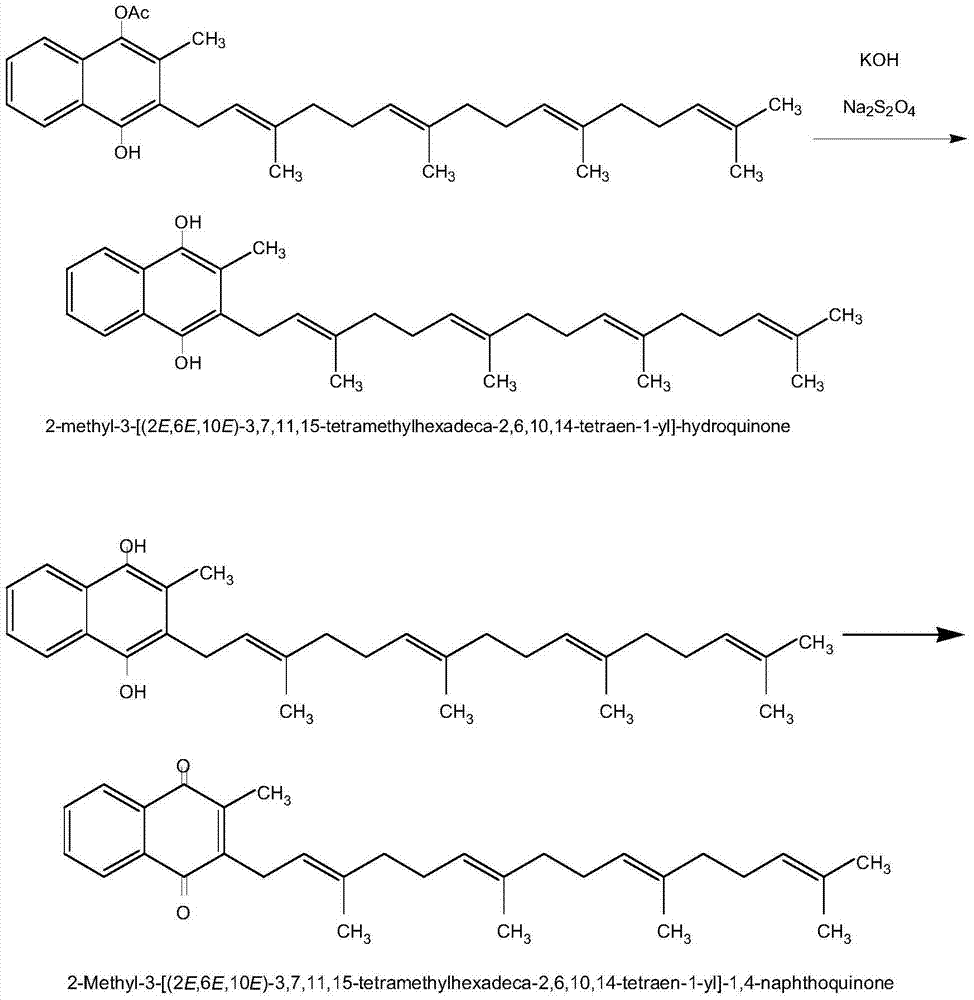
[0022] Step 2: Alcoholysis
[0023] To the above materials, add 40 ml of heptane, add 20 g of water, 10 g of potassium hydroxide, 1 g of hydrosulfite and 15 ml of methanol under stirring. Continue stirring, and test the disappearance of the intermediate product without the previous step.
[0024] The mixture was left to stand for stratification, 20 ml...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com