Method for preparing hexafluoropropylene oxide oligomer
A technology of hexafluoropropylene oxide and oligomers, which is applied in the field of preparation of hexafluoropropylene oxide oligomers, can solve the problems of low reaction conversion rate, low average molecular weight, and difficulties, and achieve simple operation process and high molecular weight distribution Low, less environmental pressure effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
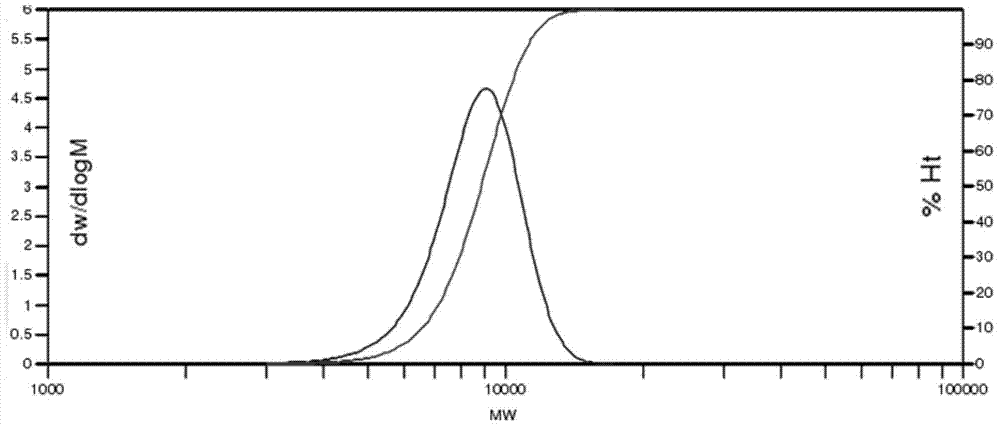
[0047] Add 65ml of tetraethylene glycol dimethyl ether, 33.17g of perfluoro-2-methyl-3-oxahexanoyl fluoride and 6.972g of finely ground KF into a 250ml single-necked round-bottomed flask through strict drying, and then Stir at room temperature for 24 hours in a glove box or under a high-purity nitrogen protection environment, and obtain light yellow homogeneous perfluoroether alkoxide after filtration. The prepared alkoxide is quickly transferred into a 1L stainless steel reaction kettle under the condition of isolating air contact and vacuumized, and the reaction kettle is started to stir. After passing through the hexafluoropropylene oxide gas purified by the drying tower, it can be observed that the temperature in the reactor starts to rise and then returns to normal state. After passing 450g of hexafluoropropylene oxide gas, the reaction is continued for 12 hours. After the polymerization reaction was completed, the reaction mixture was taken out using a two-necked round-b...
Embodiment 2
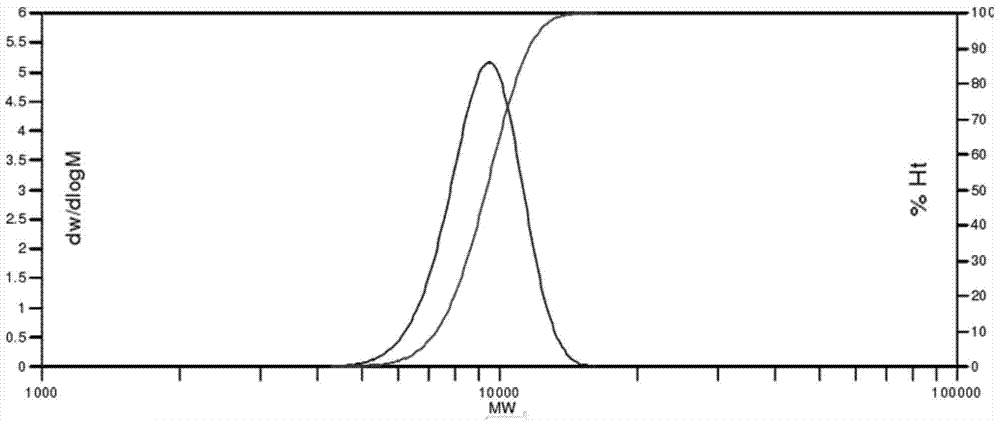
[0049] 65ml of tetraethylene glycol dimethyl ether, 33.17g of perfluoro-2-methyl-3-oxahexanoyl fluoride, 20ml of meta-trifluoromethylbenzene and 6.972g of KF Add it into a 250ml single-necked round-bottom flask, then place it in a glove box or under a high-purity nitrogen protection environment and stir at room temperature for 24 hours, and obtain a pale yellow homogeneous perfluoroether alkoxide after filtration. The prepared alkoxide is quickly transferred into a 1L stainless steel reaction kettle under the condition of isolating air contact and vacuumized, and the reaction kettle is started to stir. Introduce the hexafluoropropylene oxide gas purified by the drying tower, and it can be observed that the temperature in the reactor starts to rise and then returns to the normal state, and the amount of hexafluoropropylene oxide is 450g, and then continue to react for 12 hours. After the polymerization reaction was completed, the reaction mixture was taken out using a two-necke...
Embodiment 3
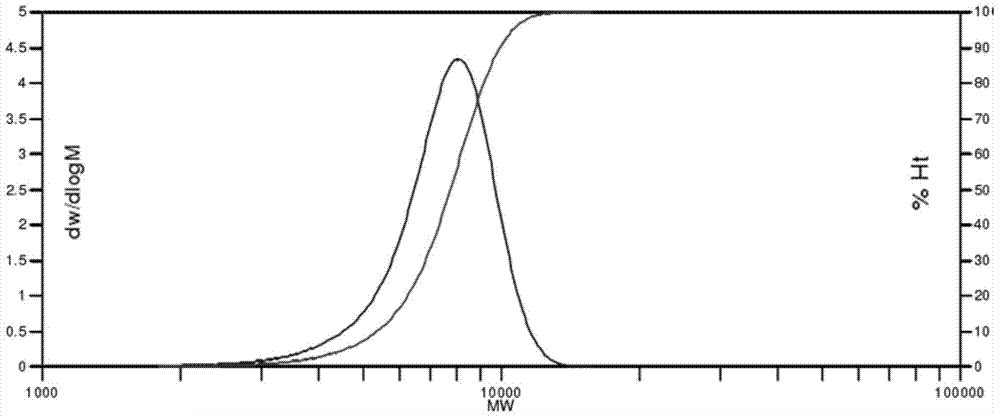
[0051] 65ml of tetraethylene glycol dimethyl ether, 33.17g of perfluoro-2-methyl-3-oxahexanoyl fluoride, 20ml of m-ditrifluoromethylbenzene and 6.972g of KF Add it into a 250ml single-necked round-bottom flask, then place it in a glove box or under a high-purity nitrogen protection environment and stir at room temperature for 24 hours, and obtain a pale yellow homogeneous perfluoroether alkoxide after filtration. The prepared alkoxide is quickly transferred into a 1L stainless steel reaction kettle under the condition of isolating air contact and vacuumized, and the reaction kettle is started to stir. Pass through the hexafluoropropylene oxide gas purified by the drying tower, and use the advection pump to pass in 40ml of trichlorotrifluoroethane at a flow rate of 0.1ml / min. It can be observed that the temperature in the reactor begins to rise and then returns to normal State, the amount of passing through hexafluoropropylene oxide is 450g and then continue to react for 12h. ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| water content | aaaaa | aaaaa |
| water content | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com